Classification Of Matter Chart
This will prevent the chart from being cut off one side.
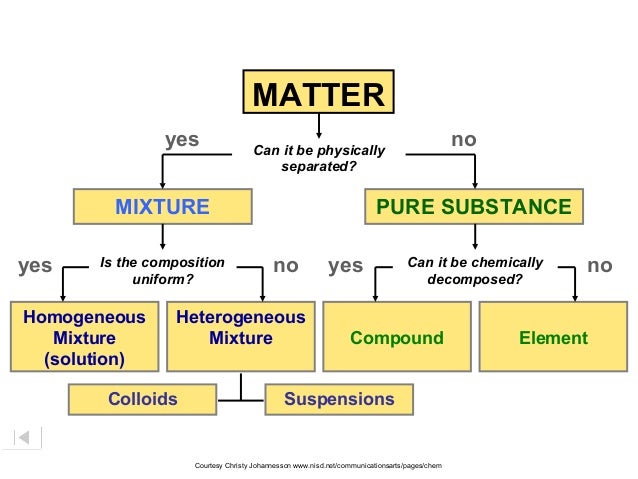
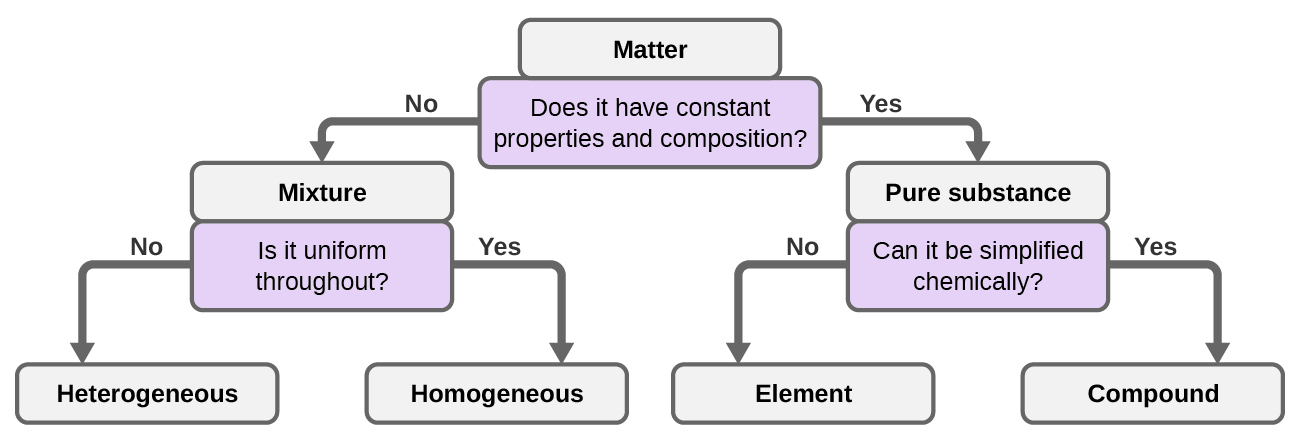
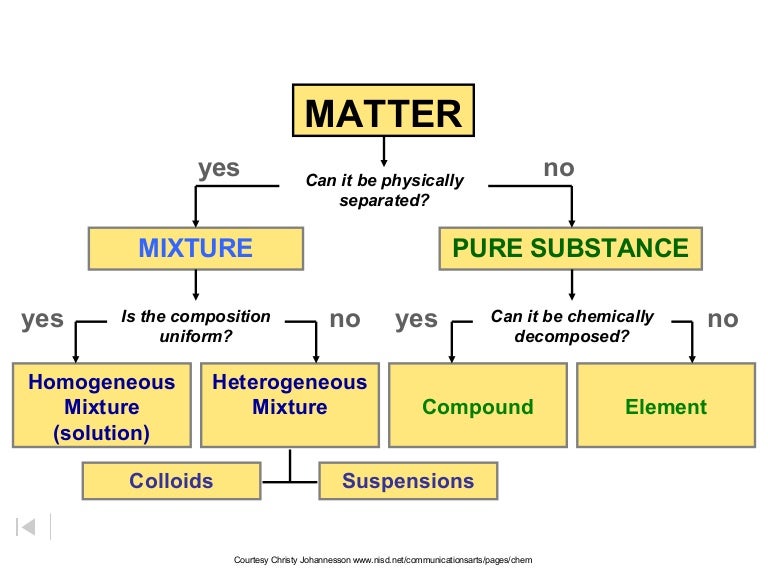
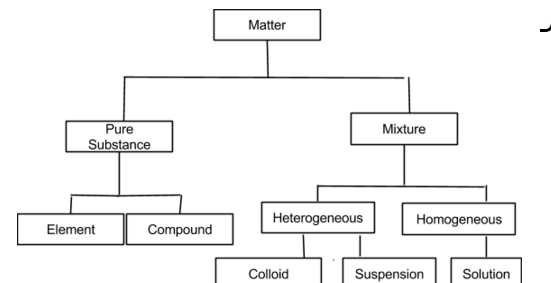
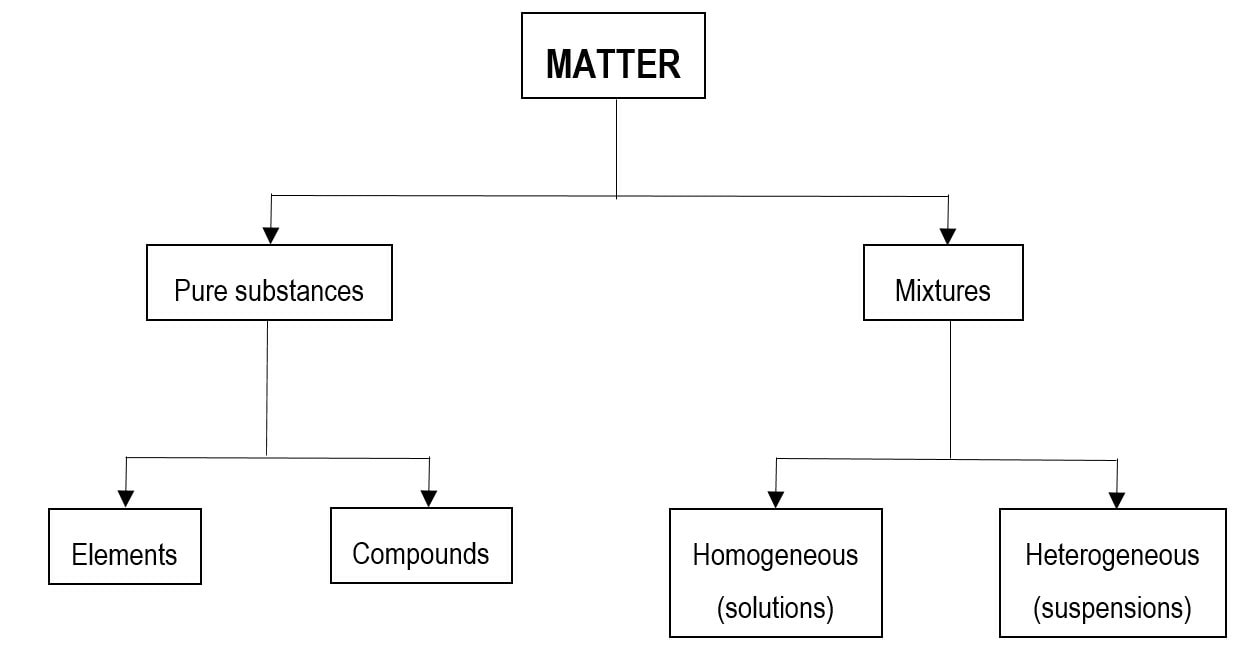
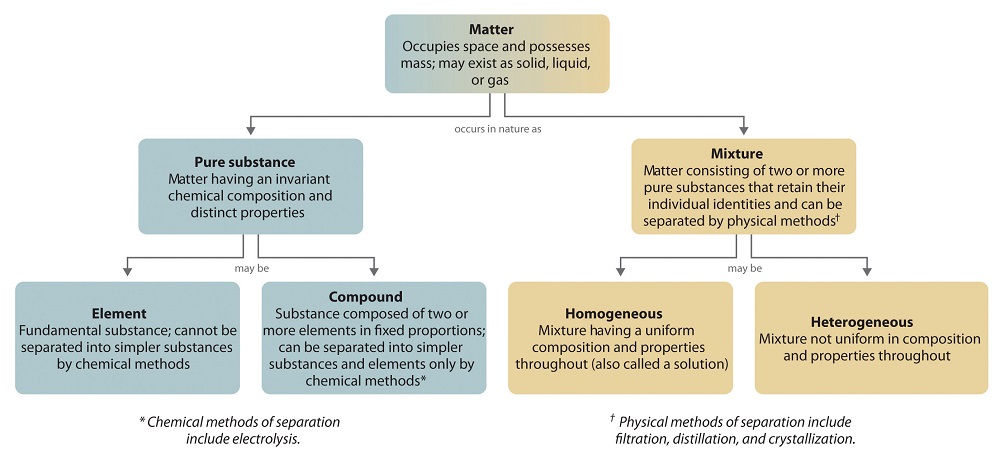
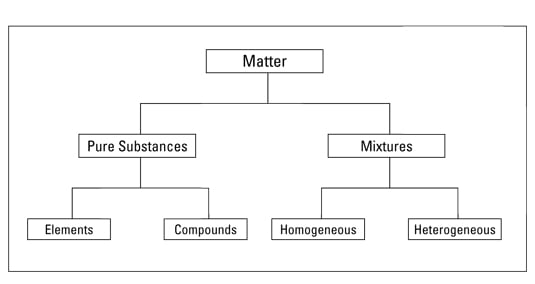
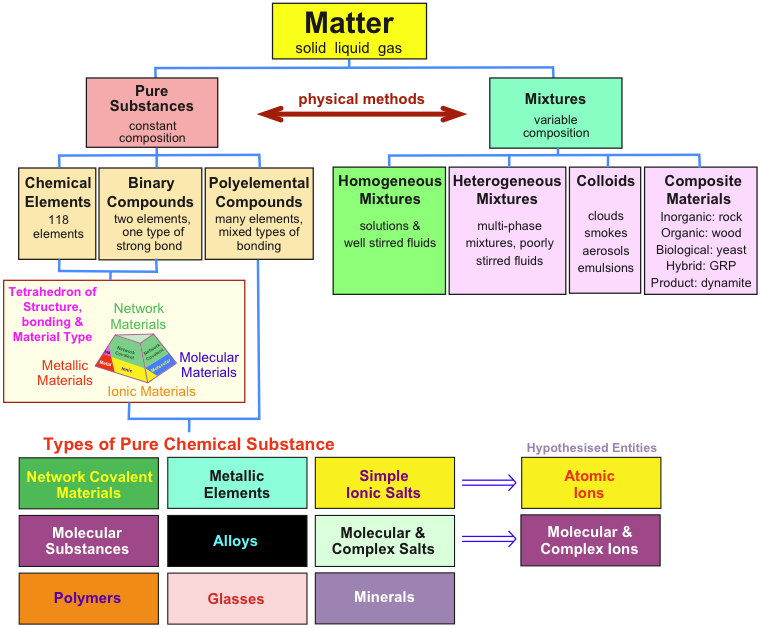
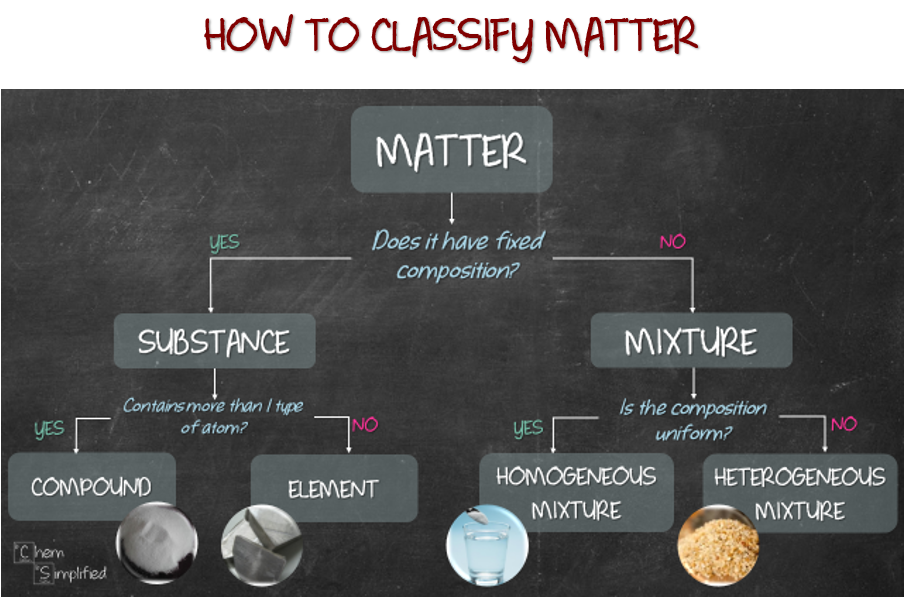
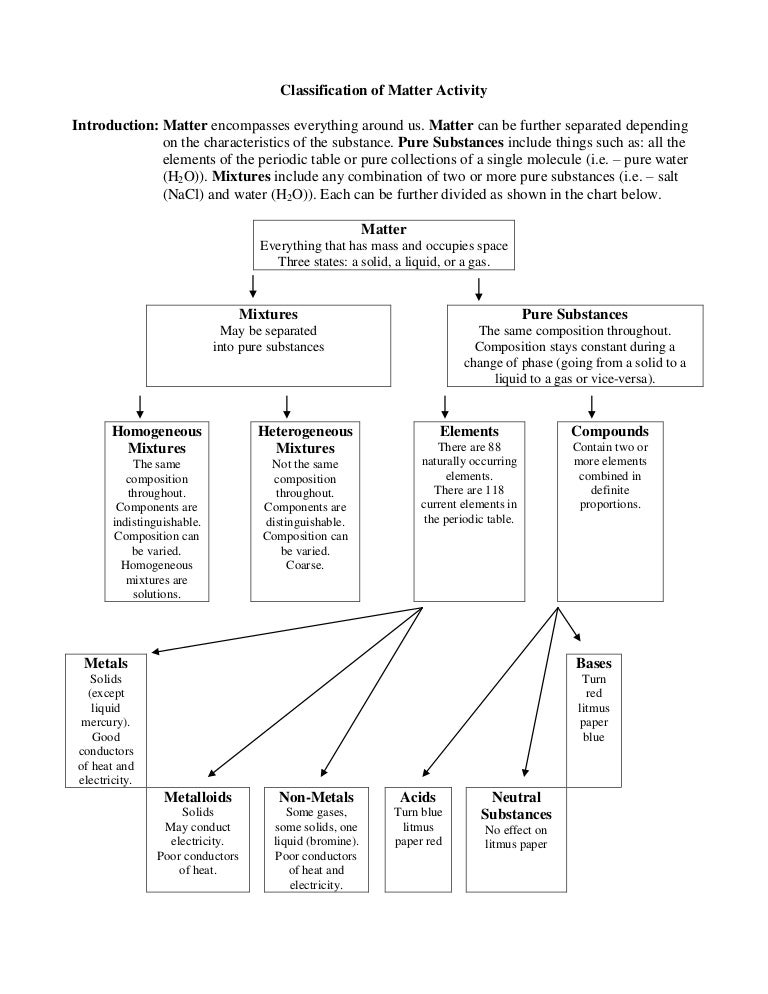
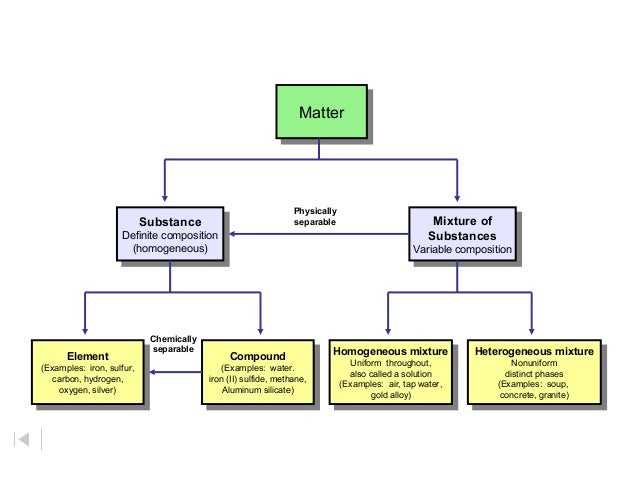
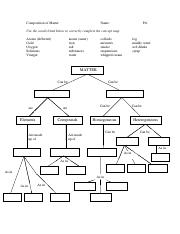
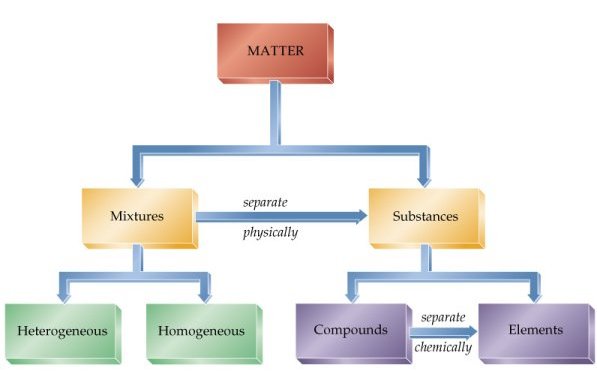
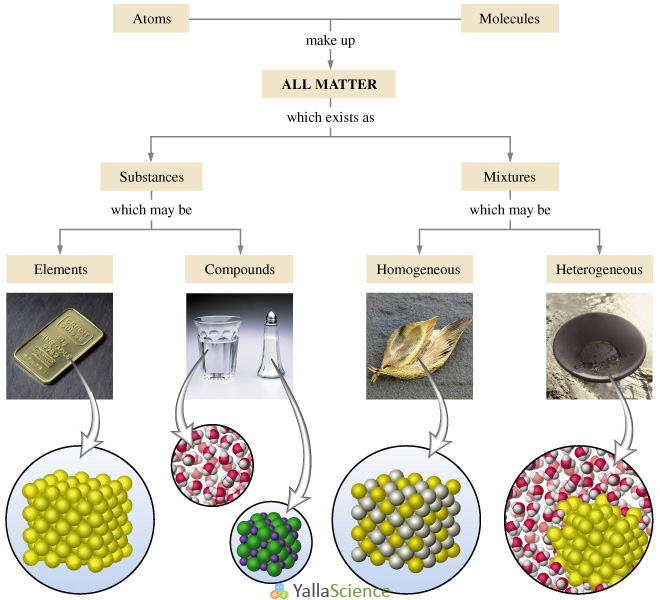
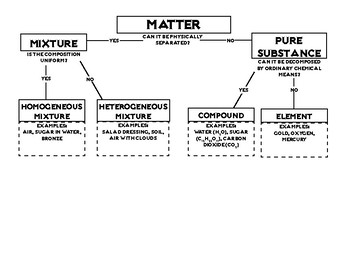
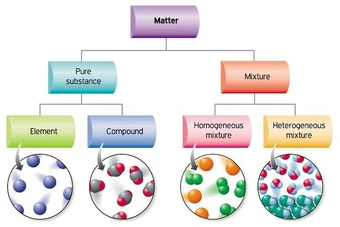
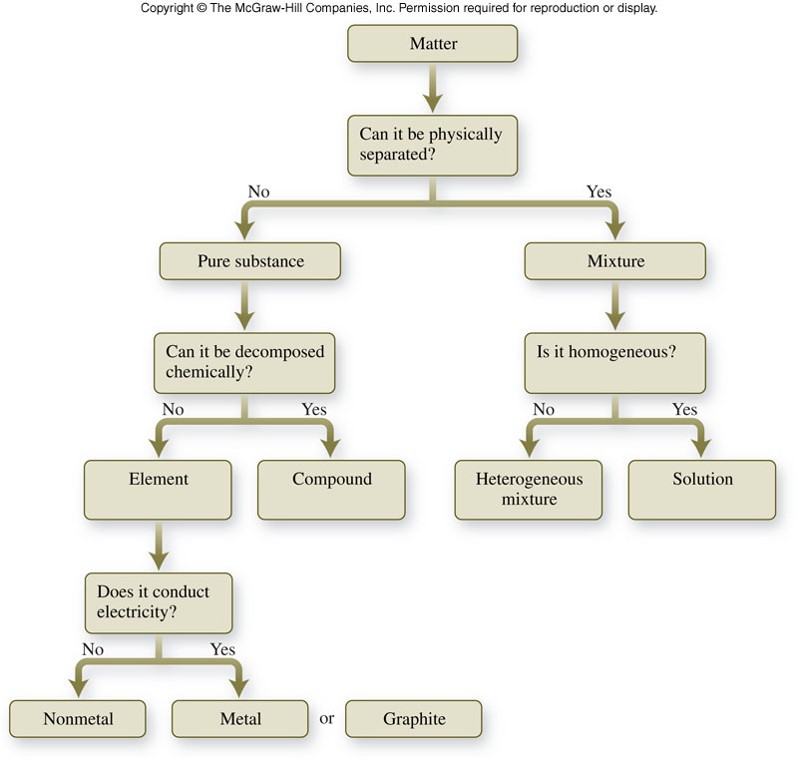
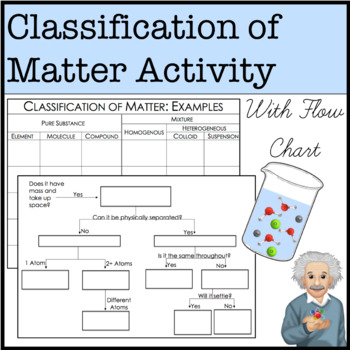
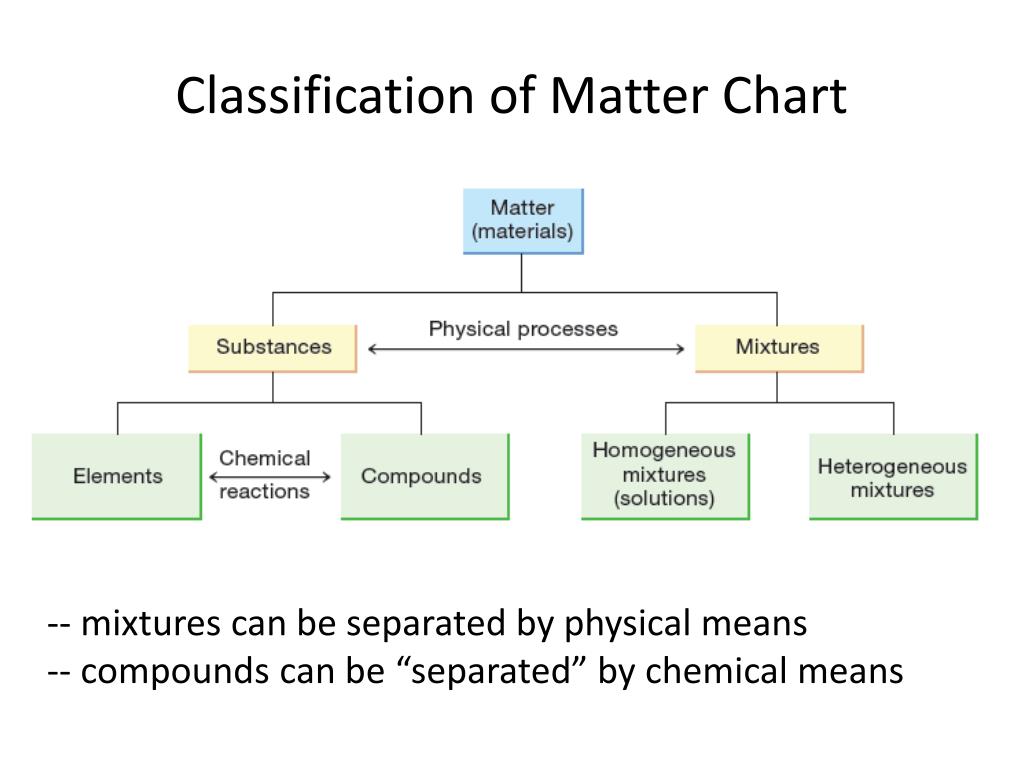
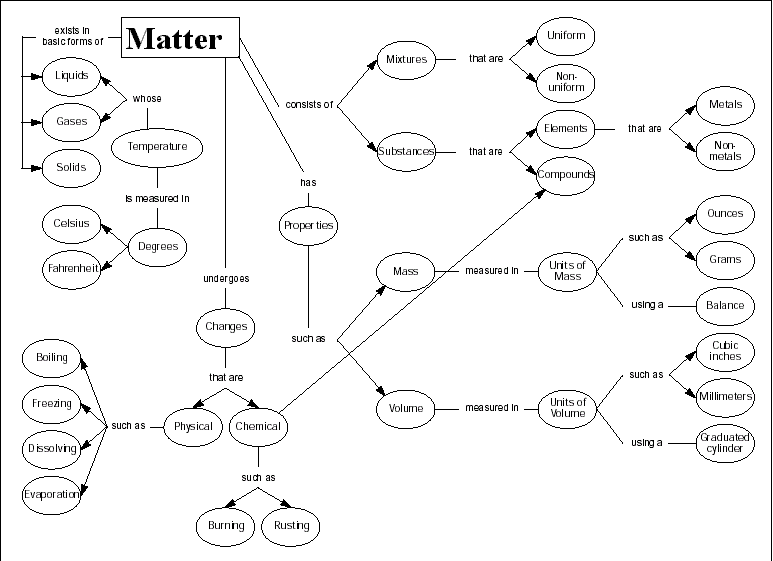
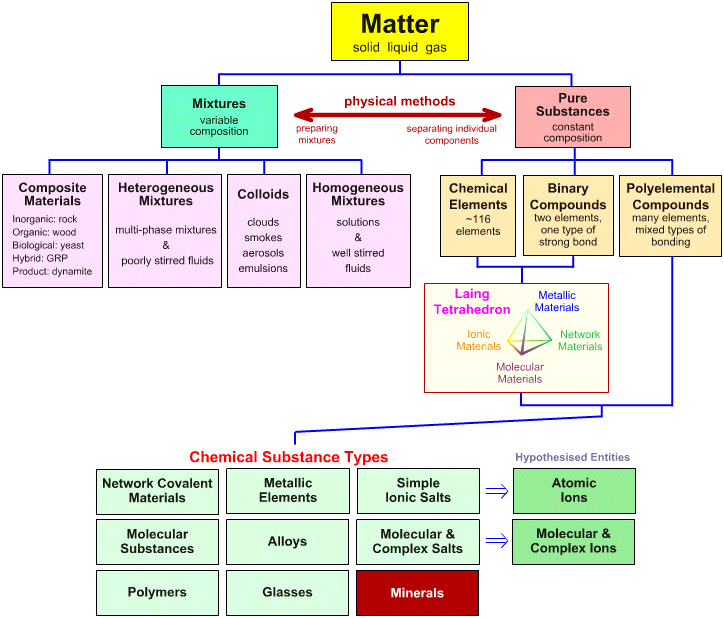
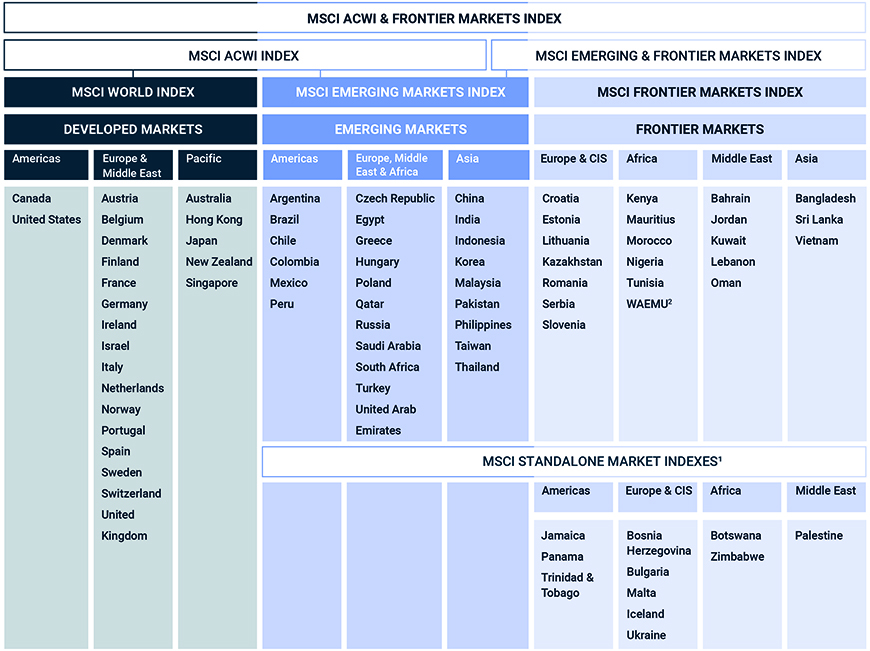
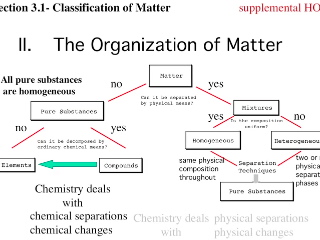
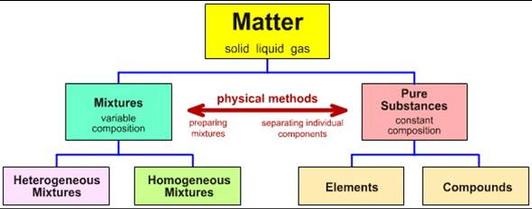
Classification of matter chart. Molecules greater than 1000 nm in size. Solid liquid or gas. A mixture is a combination of two or more substances where these substances are not bonded or joined to each other and no chemical reaction occurs between the substances.
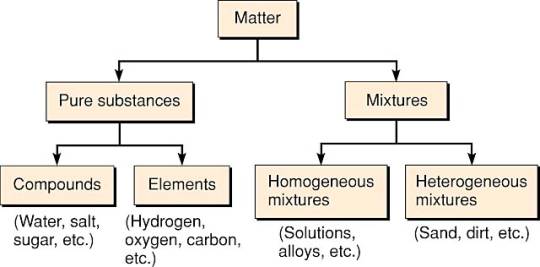
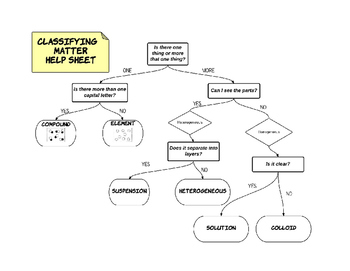
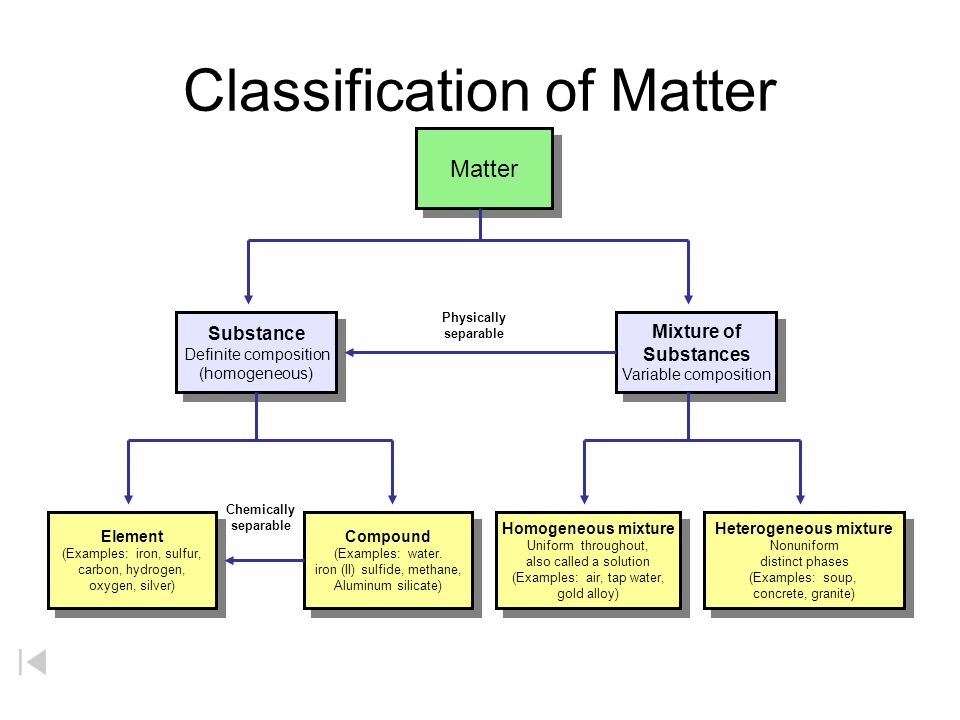
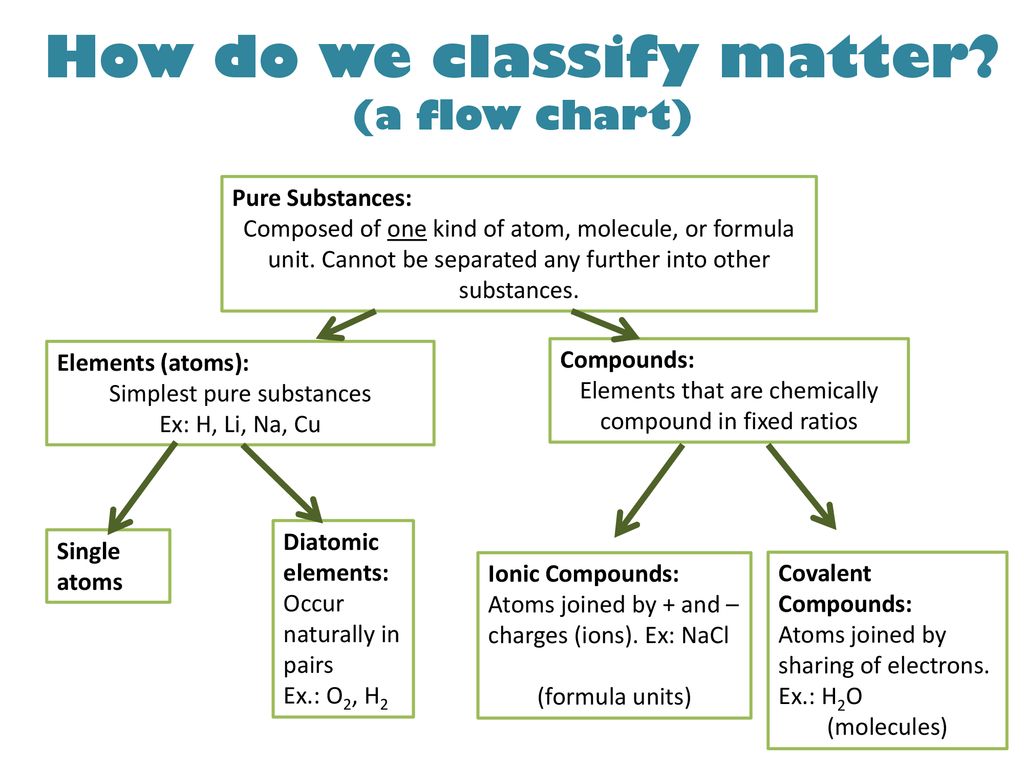
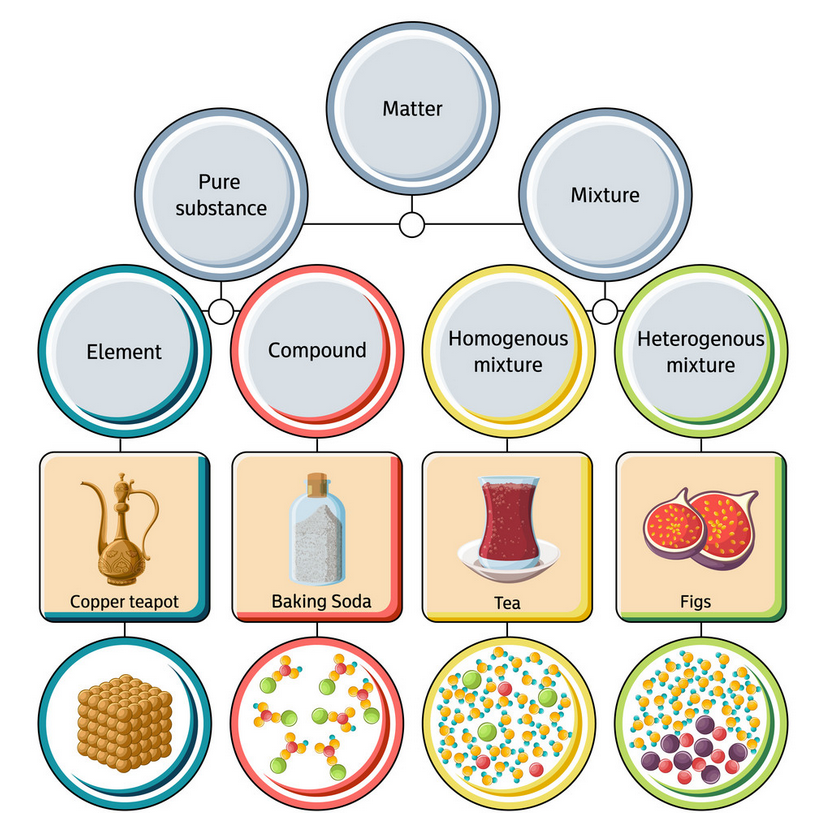
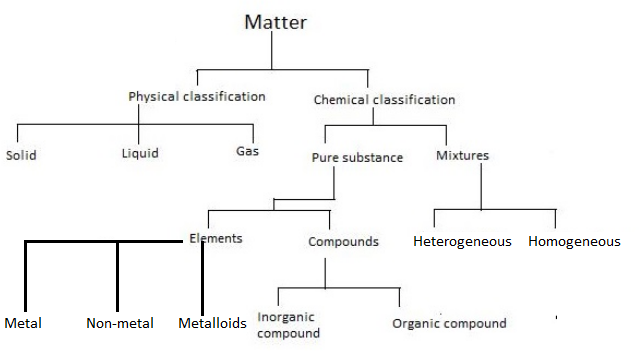
Chemical elements compounds homogeneous mixtures and heterogeneous mixtures. Classification of matter chart 38 95 after children become familiar with elements and compounds they are ready to classify matter. Solids liquids and gases.
Pause the videos when you need more time to write down observations. Molecules 2 1000 nm in size. Classification of matter flowchart.
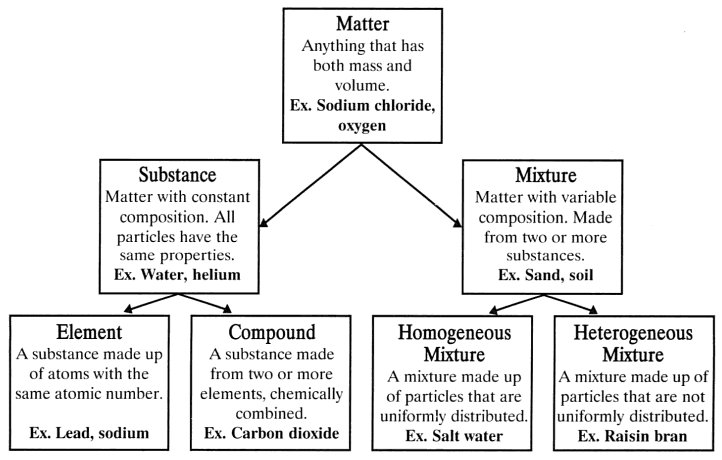
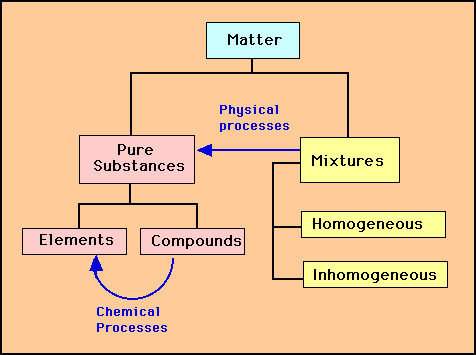
Substance is made up of elements or compounds. Because the force of gravity is considered to be the same everywhere on earth s surface 2 2 lb a weight equals 1 0 kg a mass regardless of the location of the laboratory on earth. Be sure to answer the video quiz questions at the end.
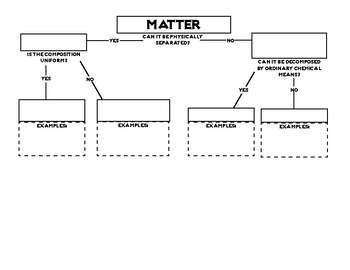
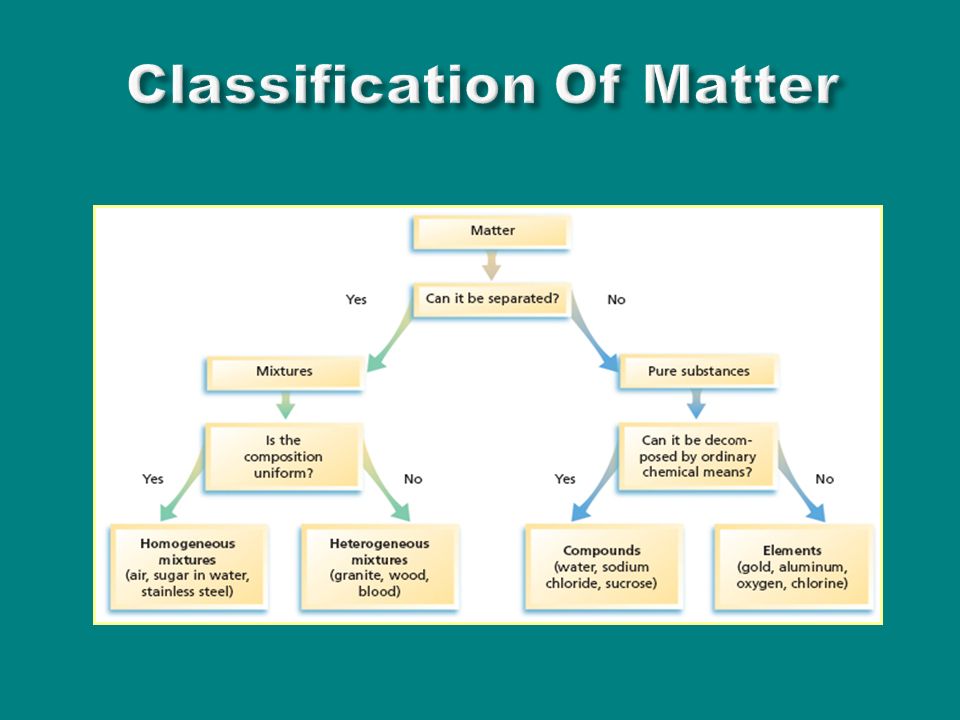
A fourth state of matter plasma occurs naturally in the interiors of stars. Classification of matter flow chart. This matter can be classified according to whether it is a mixture or a pure substance.
All the objects and substances that we see in the world are made of matter. In the classification of matter chart letter b. Molecules 0 1 2 nm in size.
Would be a n. In order to print this chart you should change your page setup to landscape. As you view the video take notes on important concepts.
A plasma is a gaseous state of matter that contains appreciable numbers of electrically charged particles figure 2. The particles are not free to move around. Liquid matter is made of more loosely packed particles.
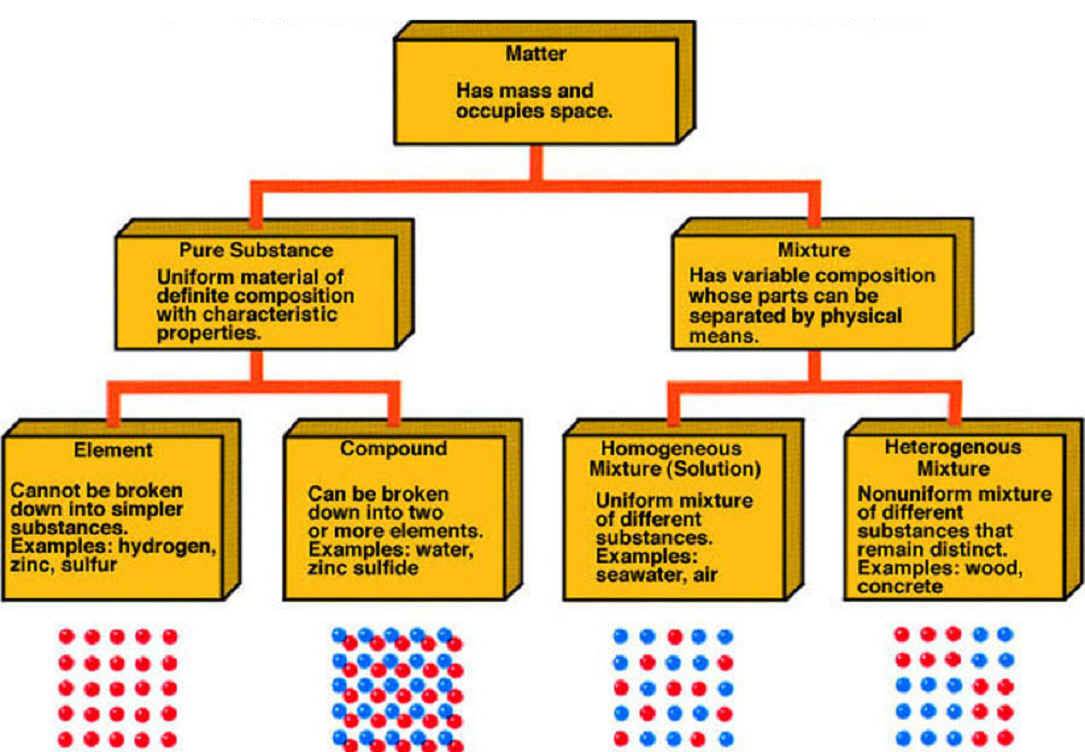
A substance is simply a pure form of matter. Watch the video from gpb below. Under normal conditions there are three distinct states of matter.
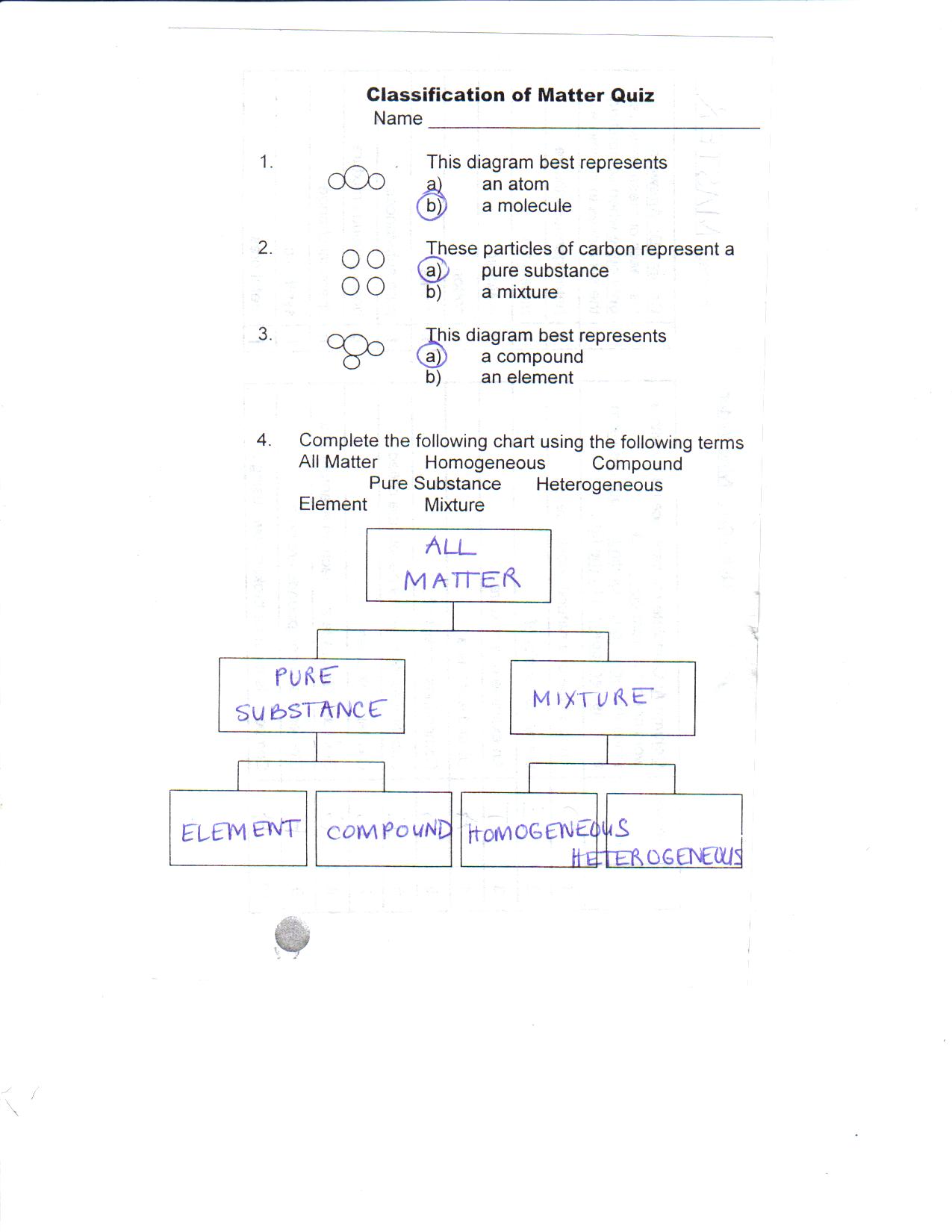
It will take the shape of its container. Now we can see in chart above matter is divided classified into two categories that are. Sixteen picture cards represent two main categories of matter pure substances and mixtures in four groups.
Matter can exist in one of three main states. A solid will retain its shape.