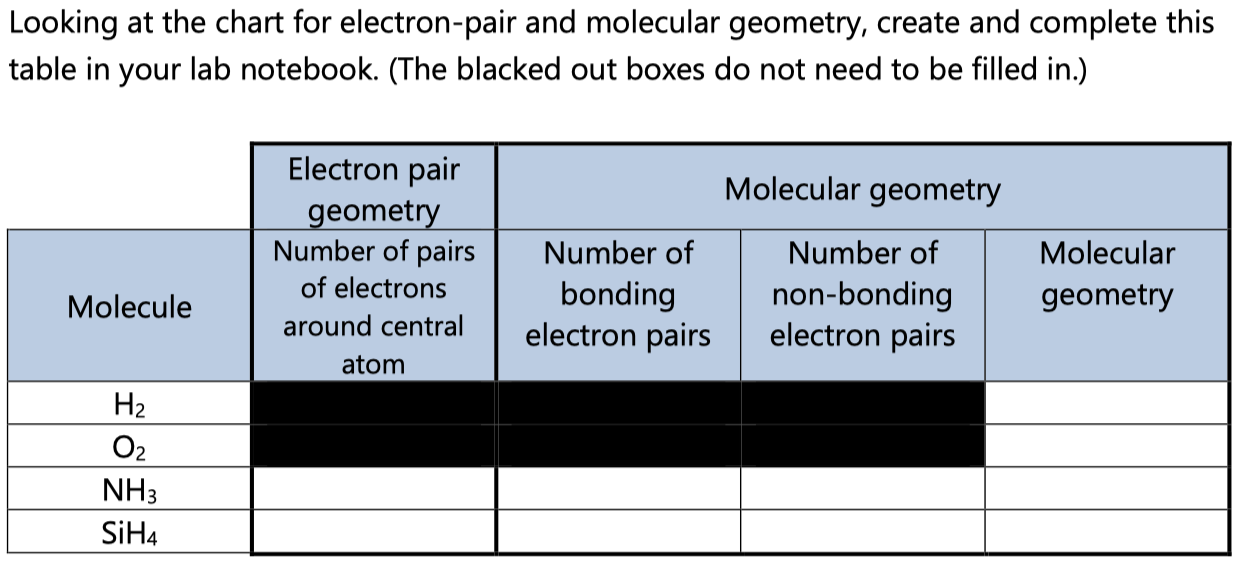
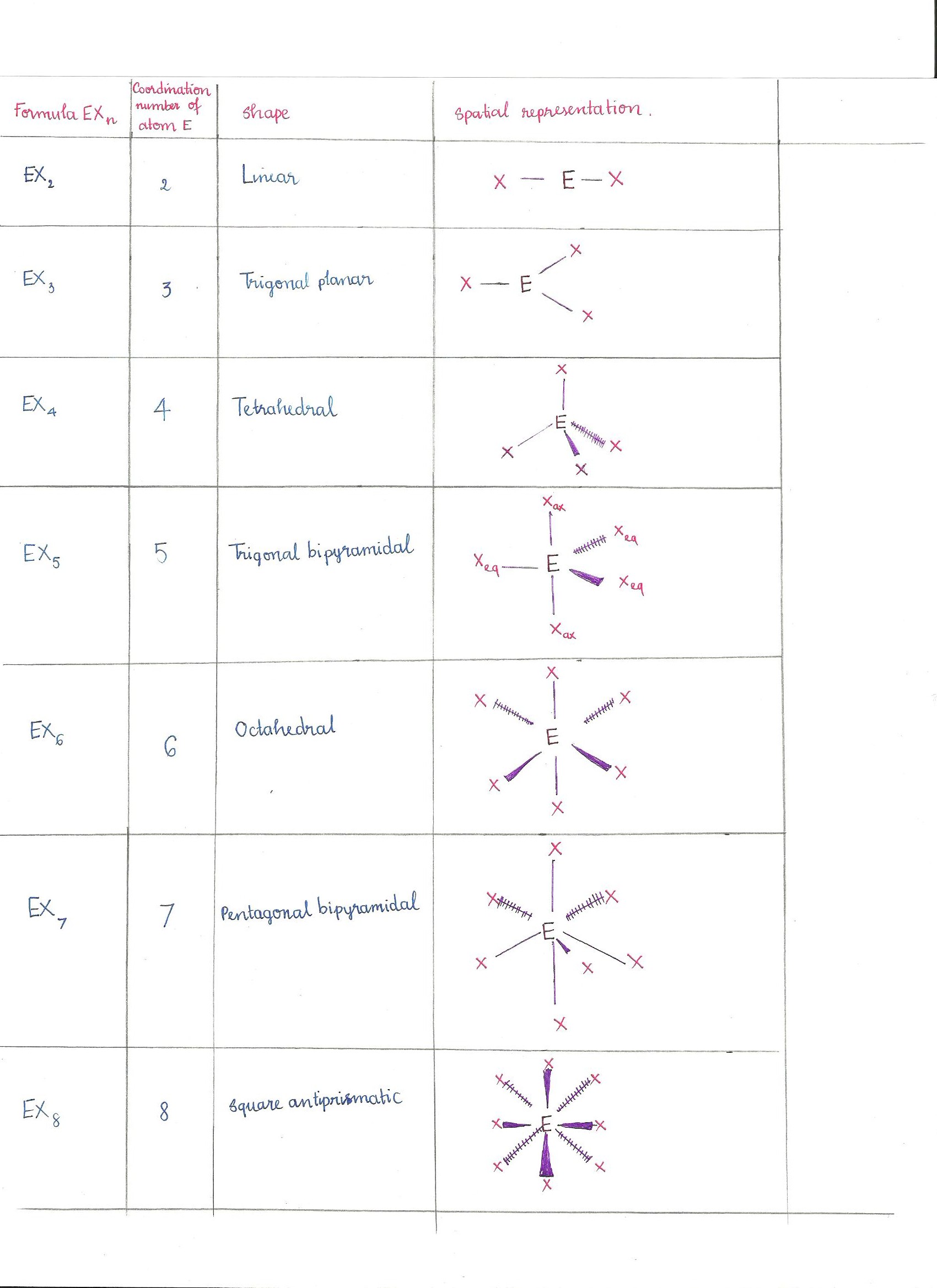
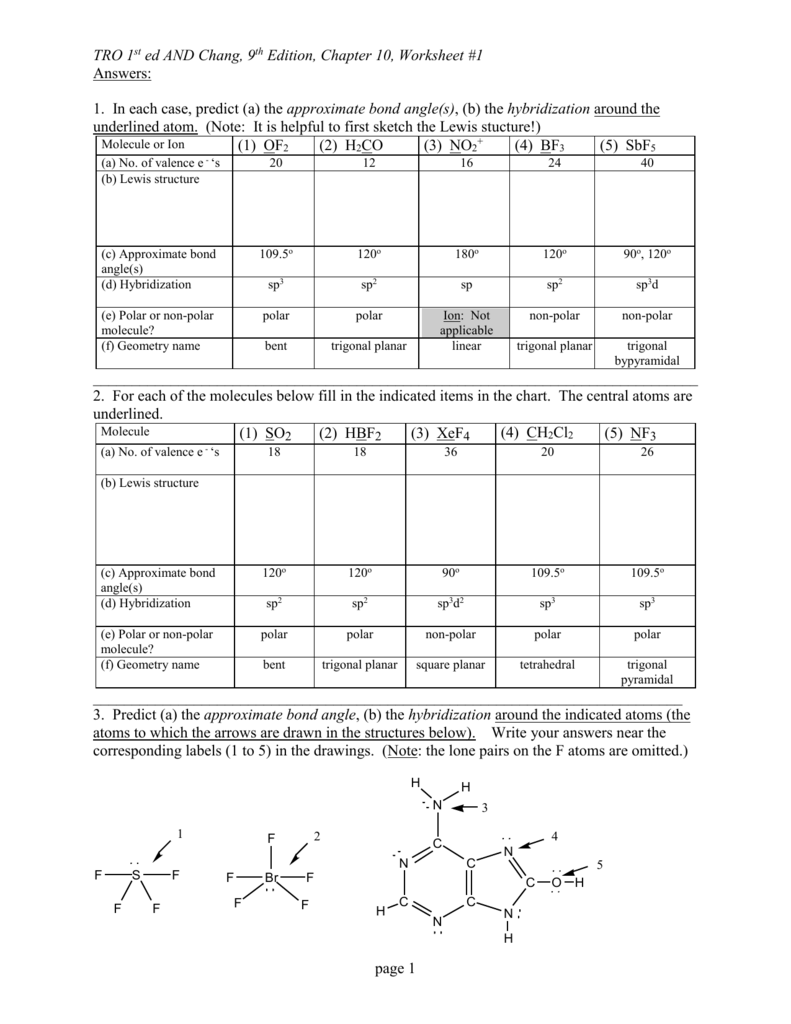
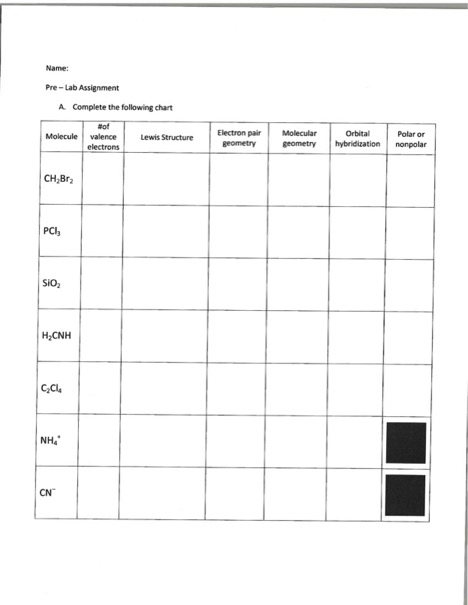
Electron Pair Geometry Chart
See graphic on middle left.
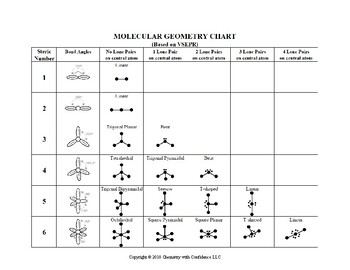
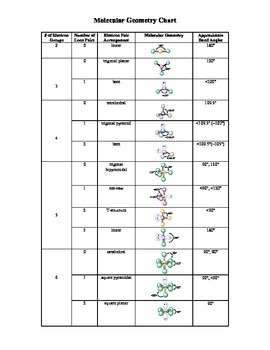
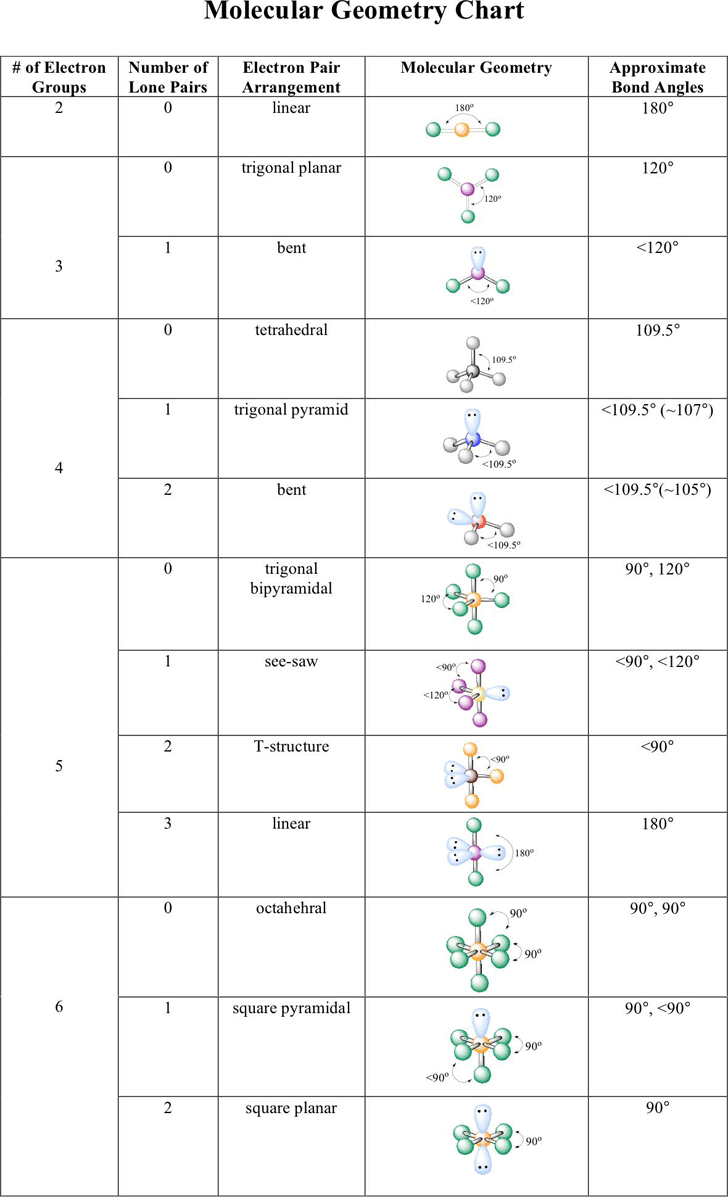
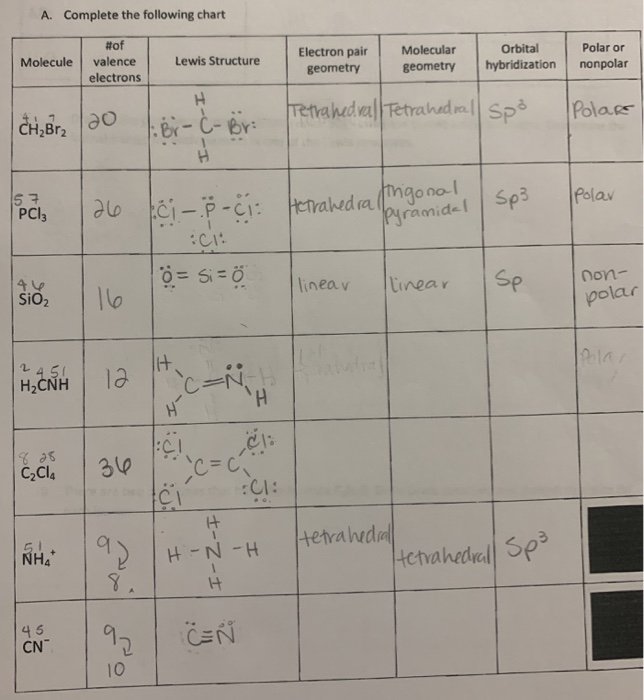
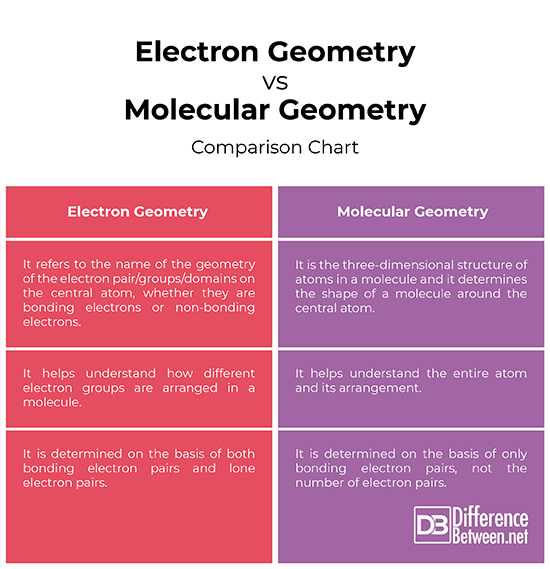
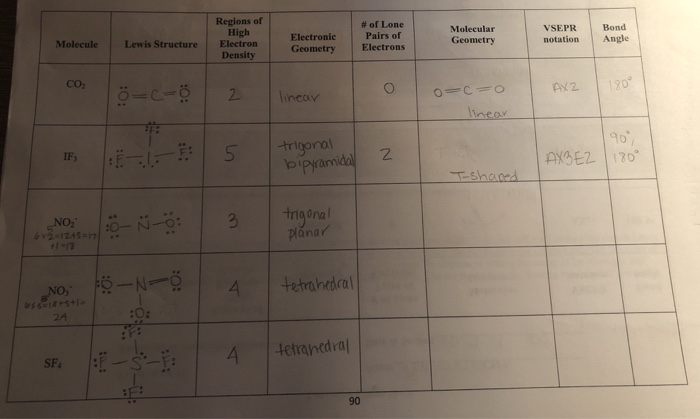
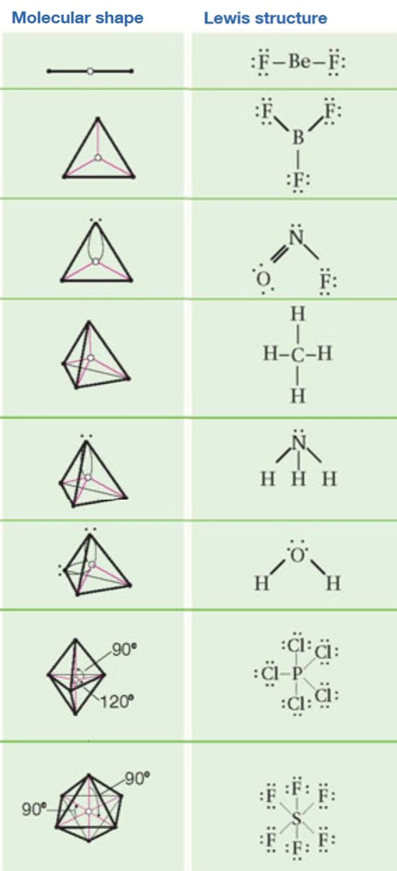
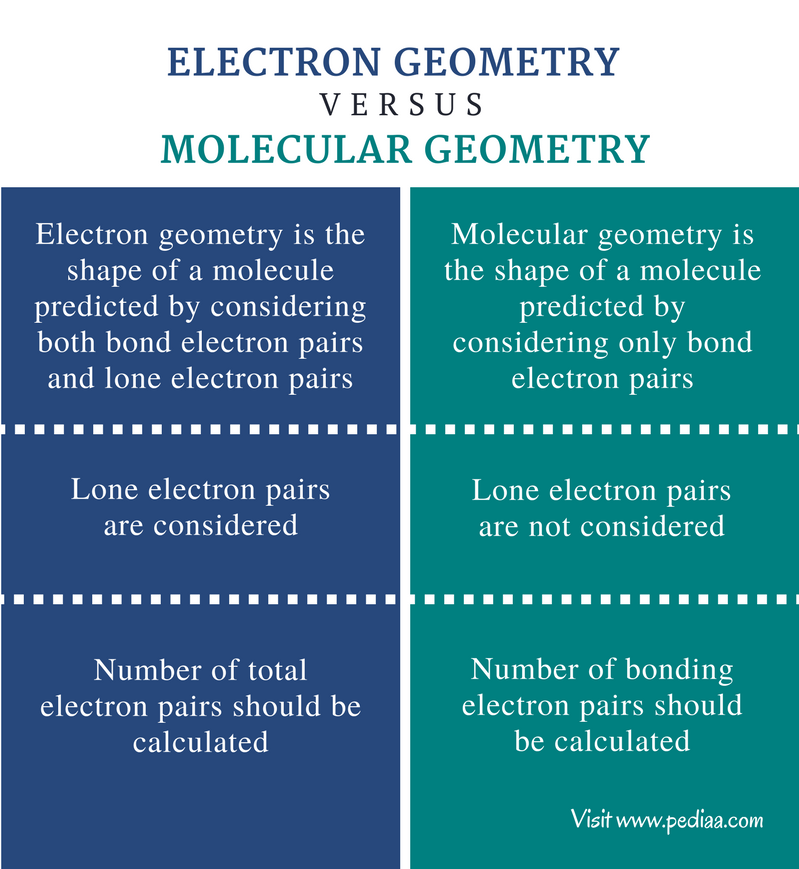
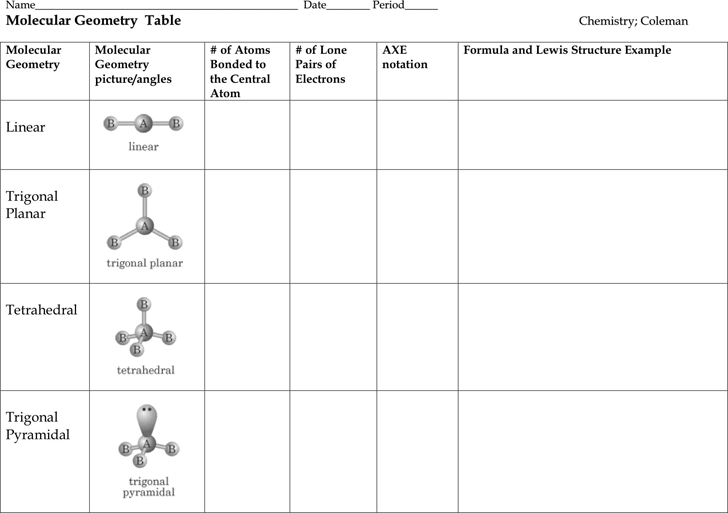
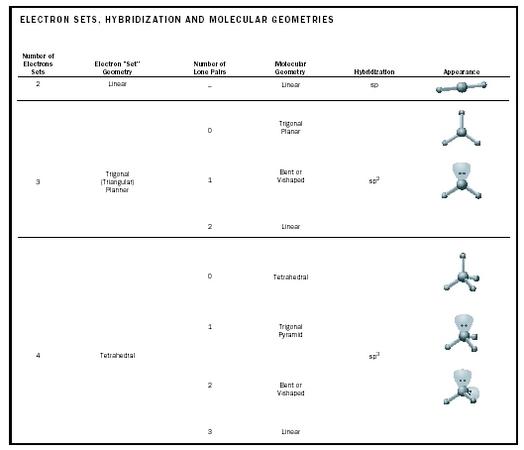
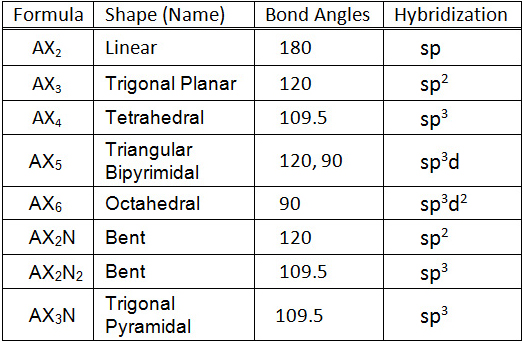
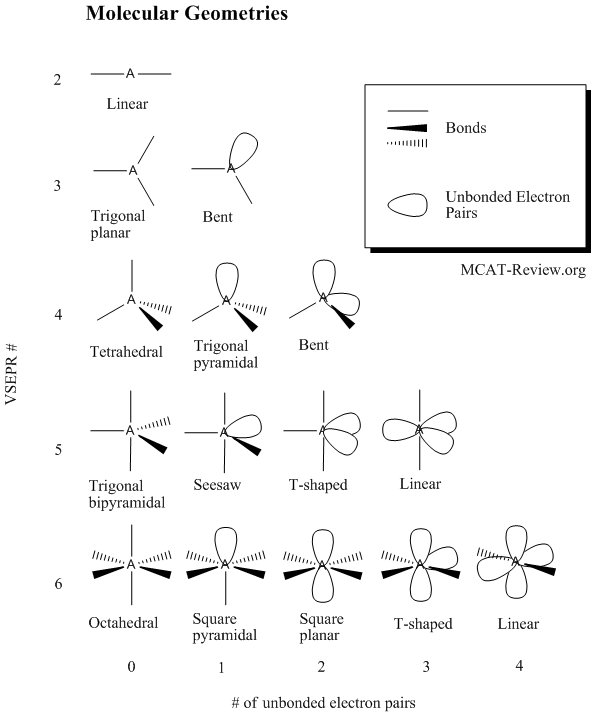
Electron pair geometry chart. In this method the geometry of a molecule is predicted by the number of valence electrons pairs around the central atom. The definitions of an electron pair is electrons that are in pairs or multiple bonds lone pairs and sometimes even just one single electron that is unpaired. The molecular geometry is the shape of the molecule.
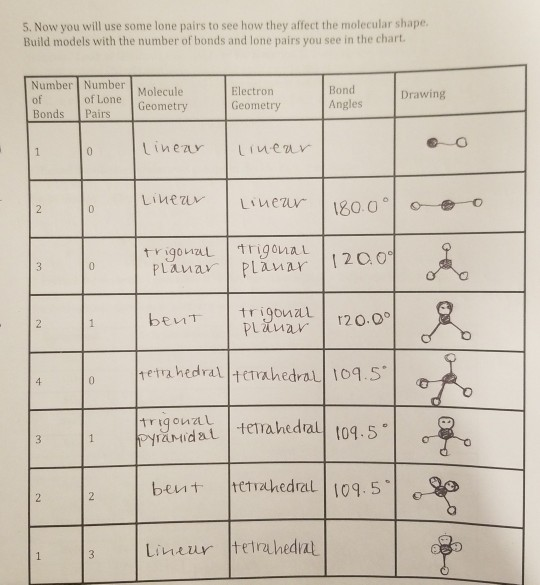
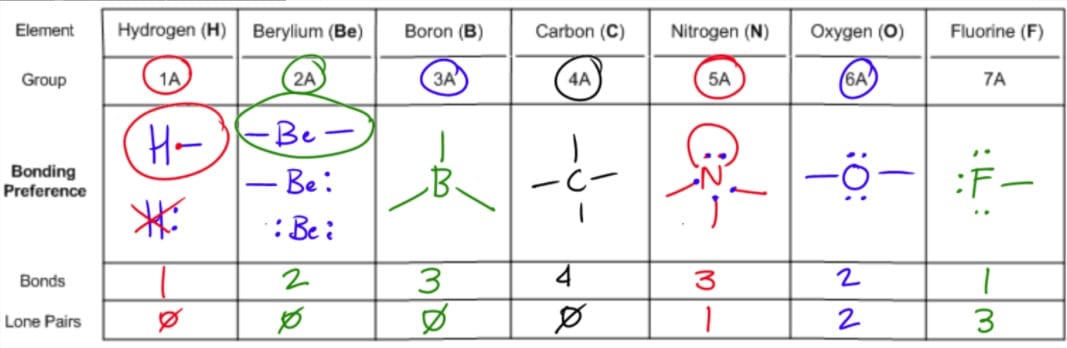
Places with bonding electrons. This applies whether they are bonding electrons or non bonding electrons. In hydrogen fluoride f as 1 bond and 3 lone pairs.
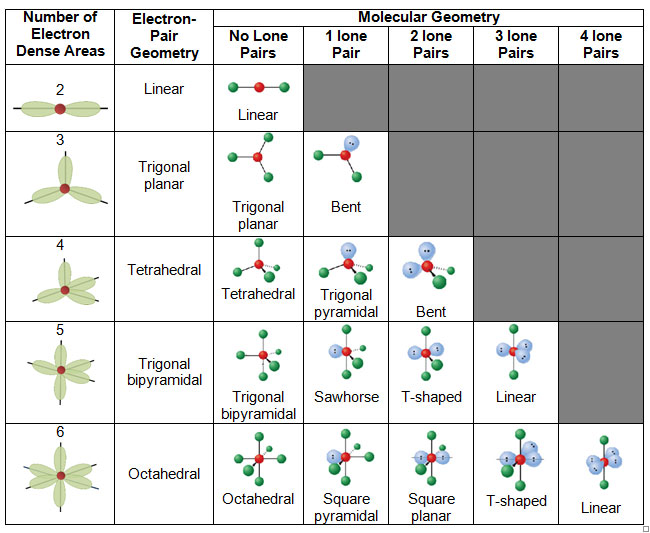
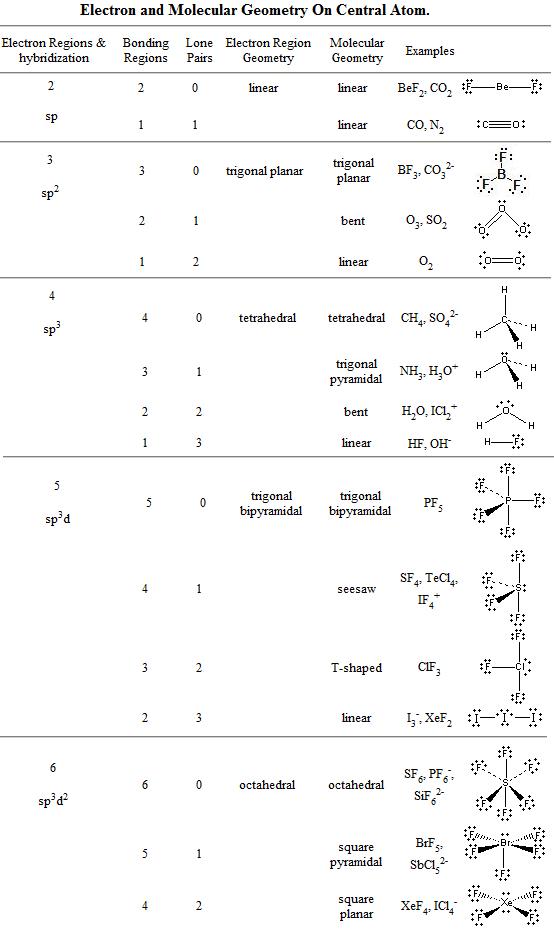
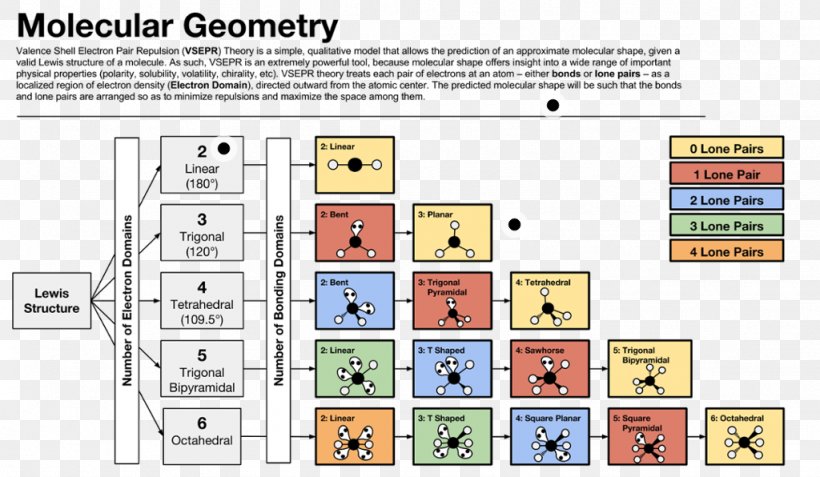
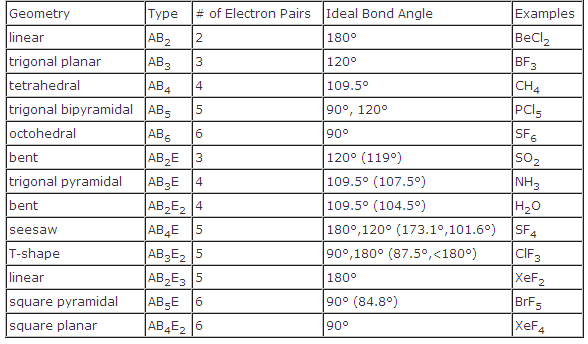
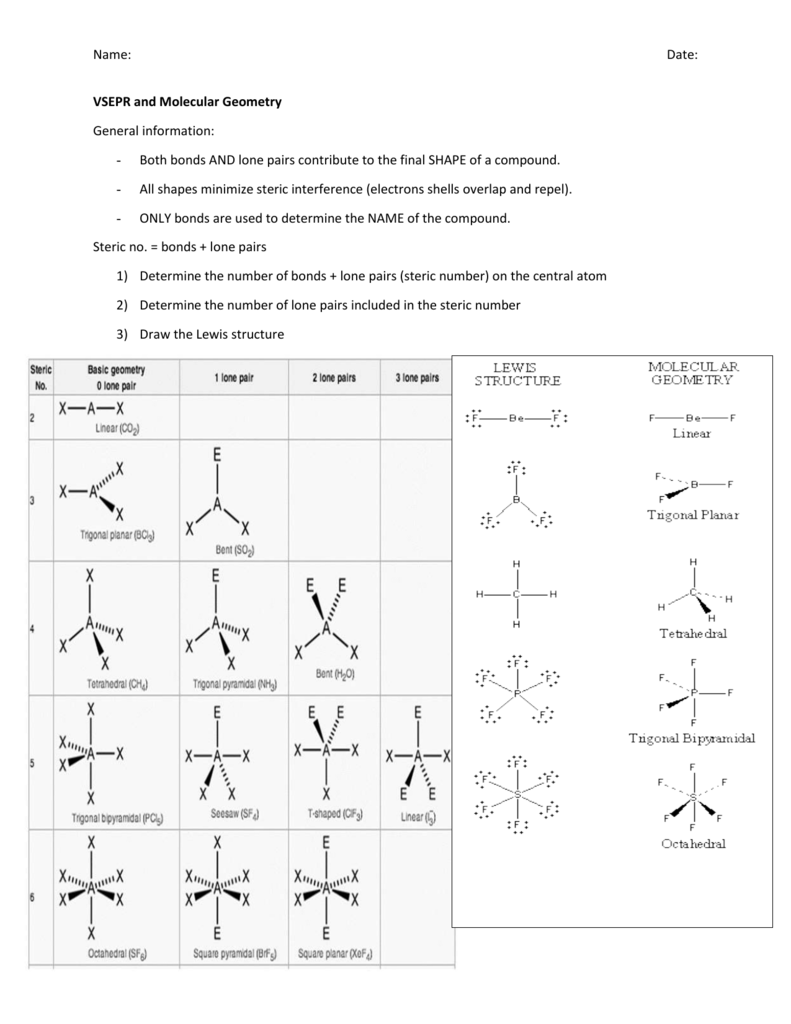
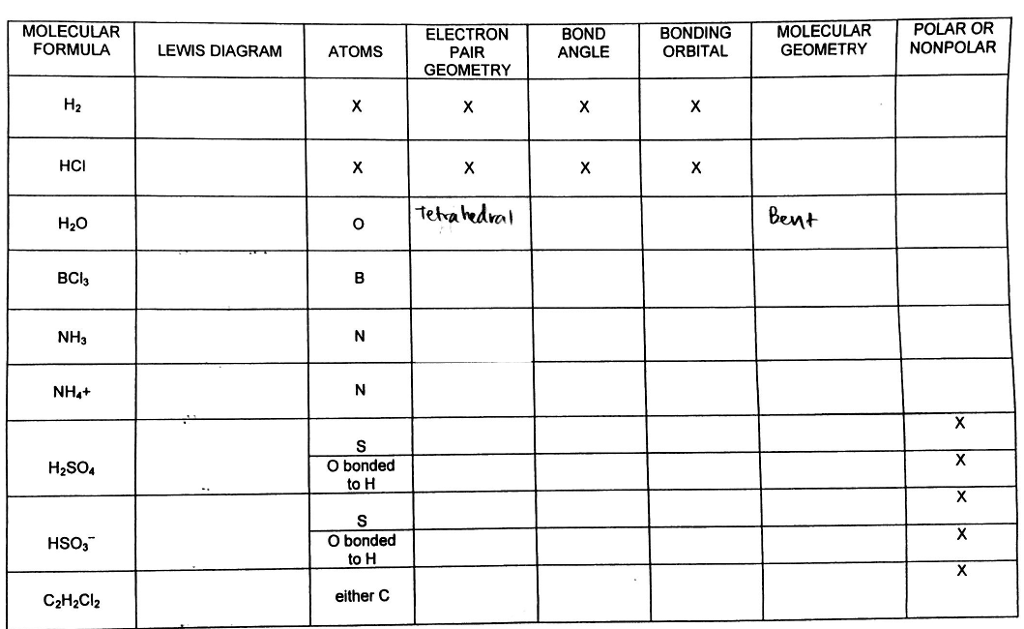
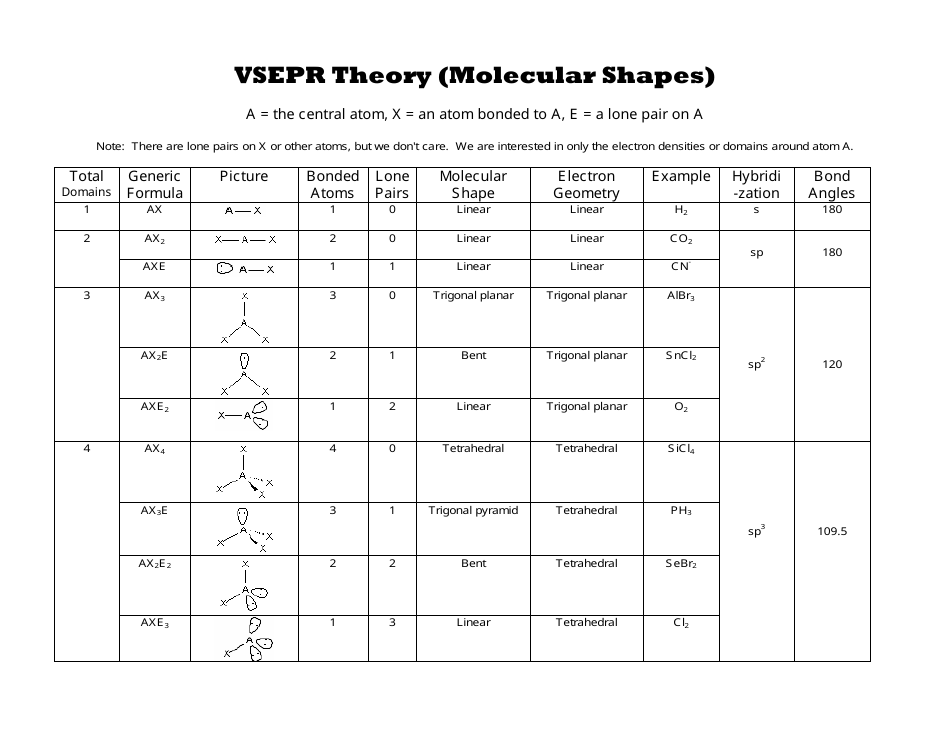
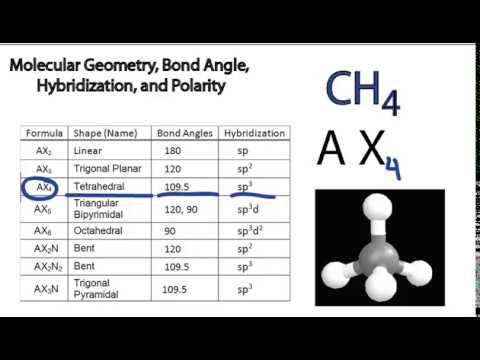
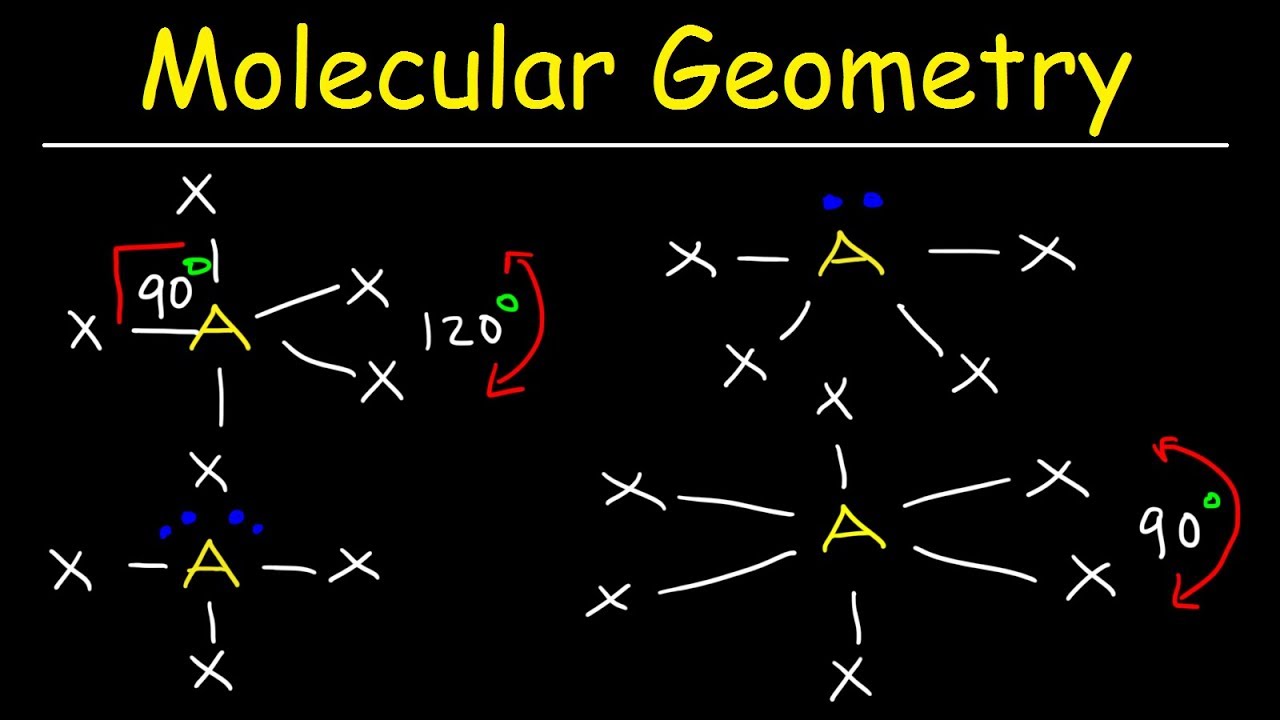
Vsepr and molecular geometry tables valence shell electron pair repulsion vsepr model lewis structures show the two dimensional distribution of atoms and electrons. The two x atoms in white are 180 away from one another. The molecular geometry or three dimensional shape of a molecule or polyatomic ion can be determined using valence shell electron pair.
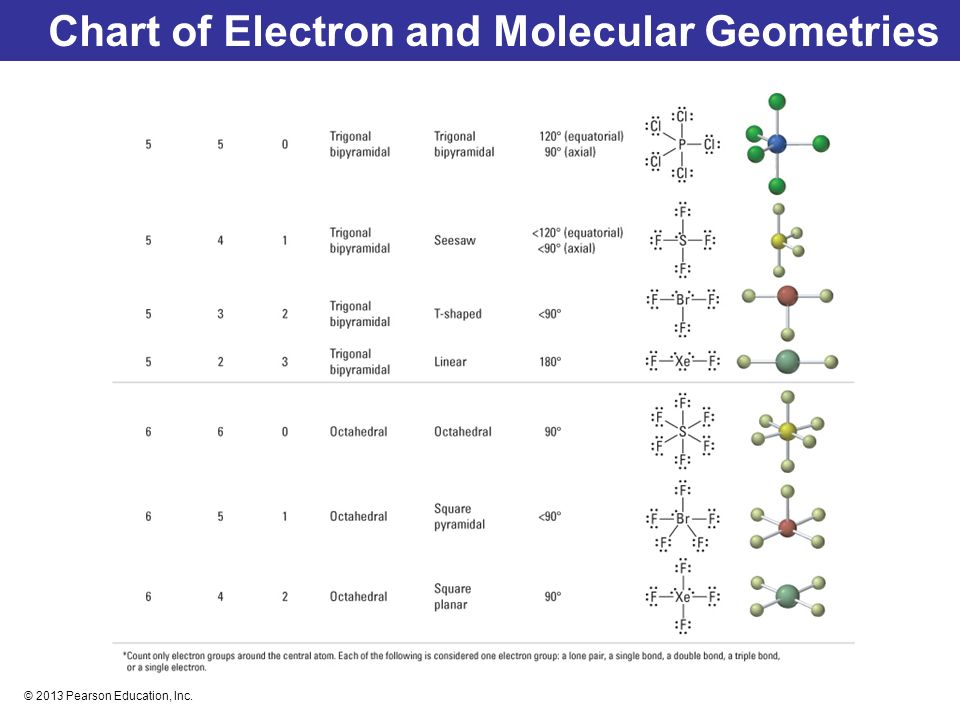
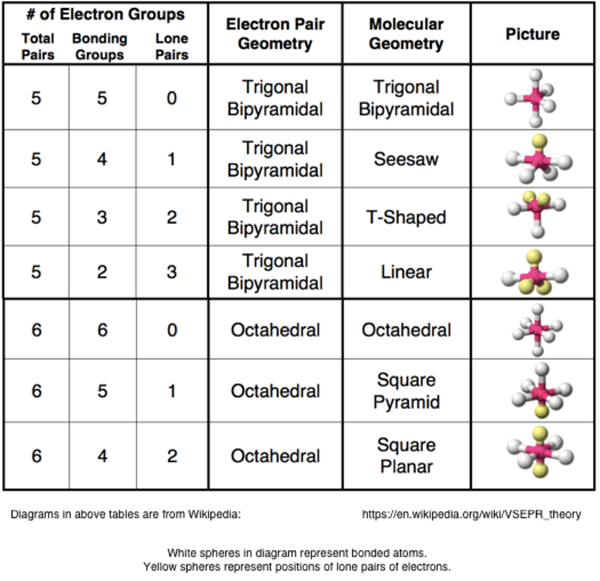
If asked for the electron pair geometry on the central atom we must respond. If the central atom also contains one or more pairs of non bonding electrons these additional regions of negative charge will behave much like those associated with the bonded atoms. This ball and stick model represents a linear compound for formula ax2.
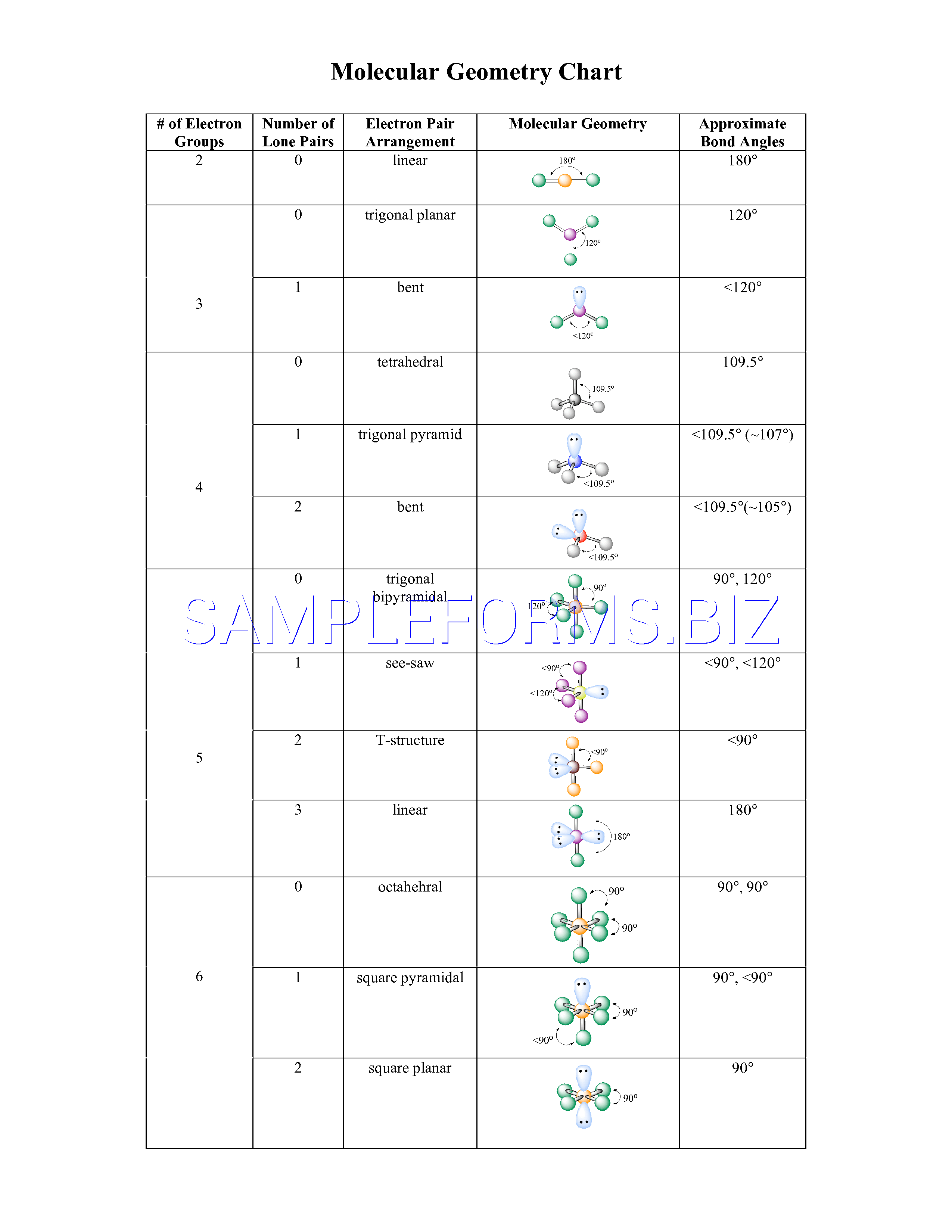
In water o has 2 bonds and 2 lone pairs of electrons. Its bond angles are 90 and 120 where the equatorial equatorial bonds are 120 apart from one another and all other angles are 90. To apply the vsepr theory we have to make some assumptions about the nature of bonding.
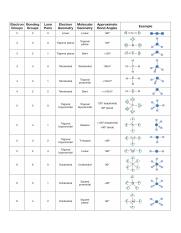
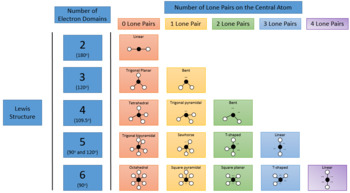
Valence shell electron pair repulsion or vsepr theory predicts the molecular geometry by this method. Find more chemistry widgets in wolfram alpha. Places with non bonding electrons.
What is electron pair geometry. In ammonia n has 3 bonds and one lone pair of electrons. In methane c has four bonds.
All have four pairs of electrons about the central atom c n o or f. Distri bution of electrons. The electron pair geometry provides a guide to the bond angles of between a terminal central terminal atom in a compound.
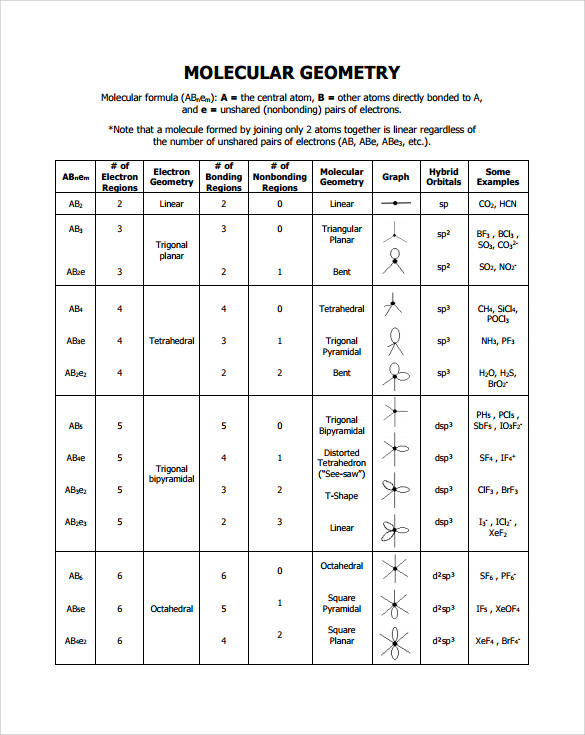
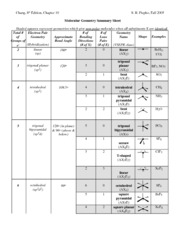
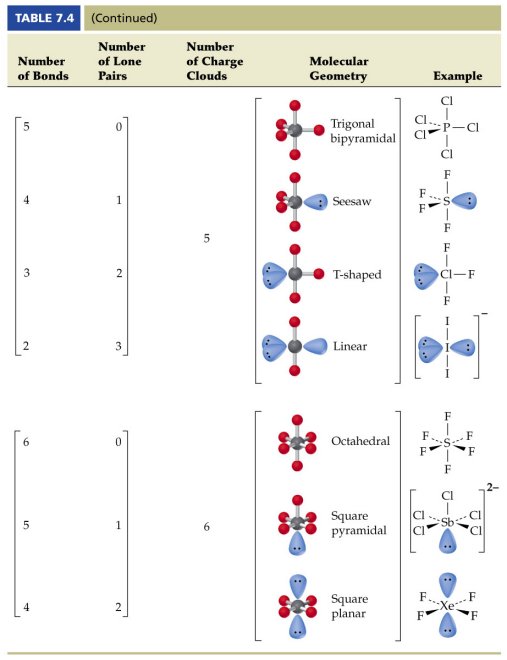
Pairs molecular shape electron geometry example hybridi zation bond angles ax 5 5 0 trigonal bipyramid trigonal bipyramid asf 5 ax 4e 4 1 see saw trigonal bipyramid seh 4 ax 3e 2 3 2 t shape trigonal bipyramid icl 3 5 ax 2e 3 2 3 linear trigonal bipyramid brf 2 sp3d 90 and 120 ax 6 6 0 octahedral octahedral secl 6 ax 5e 5 1 square pyramid octahedral if 5. Places where electrons are found. Electron geometry is the term used for the geometry of the electron pair located on the central atom.
In methane ammonia water and hydrogen fluoride the electron pair geometry is tetrahedral.