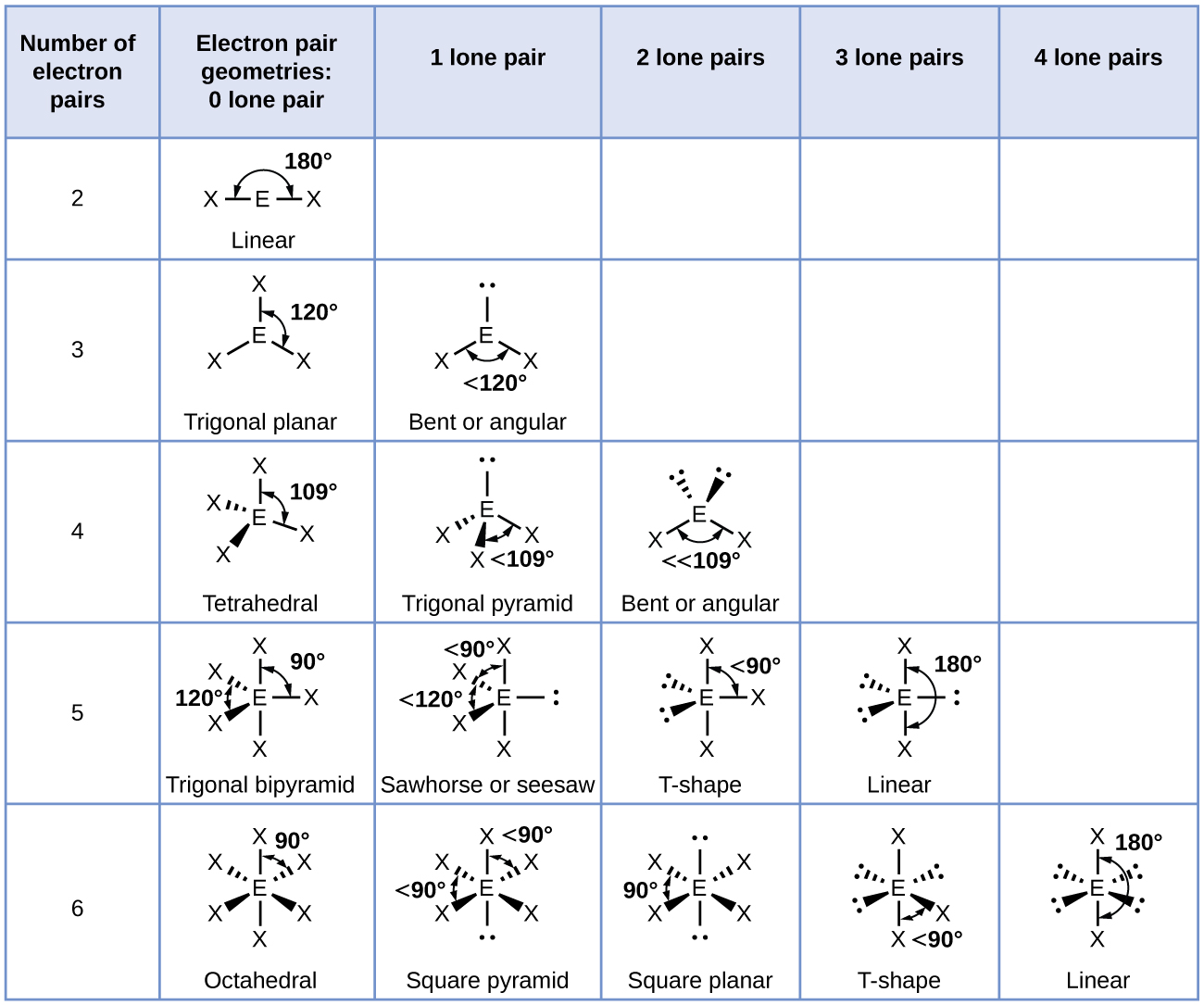
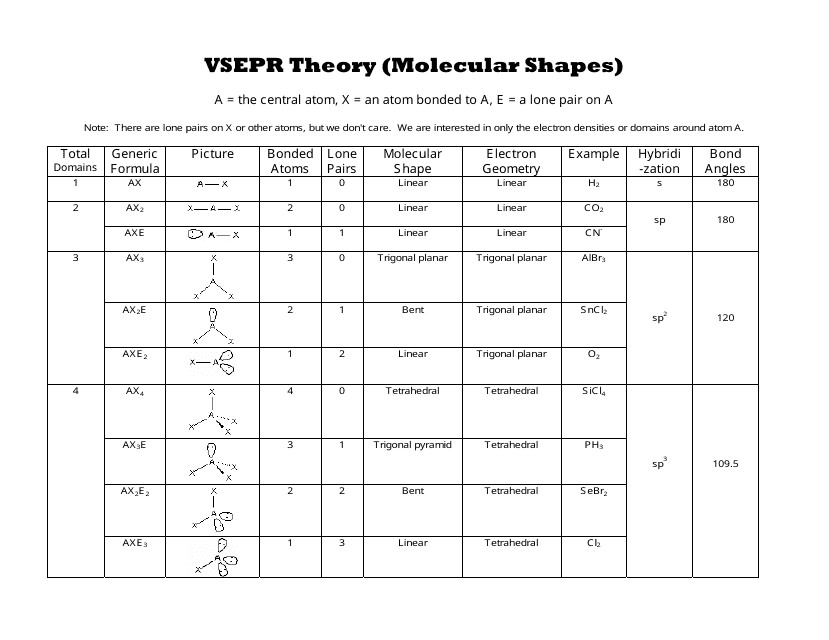
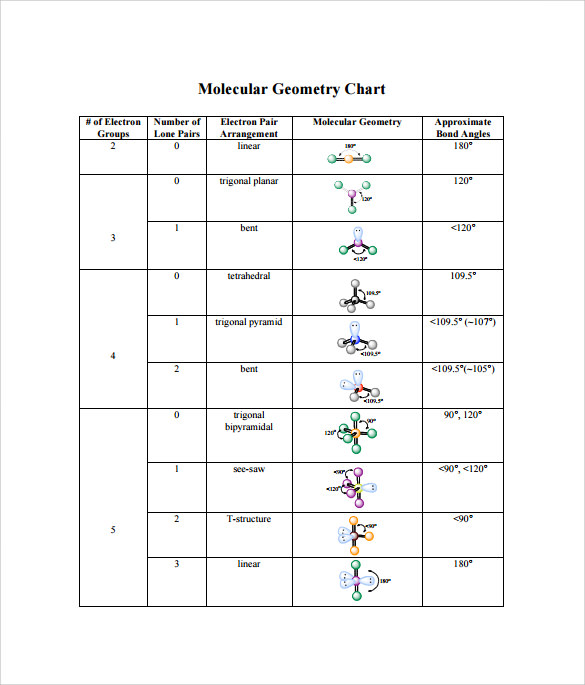
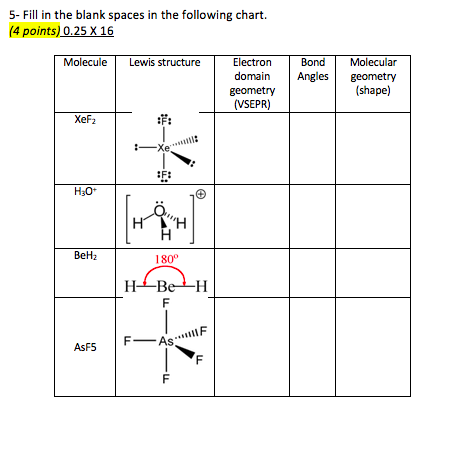
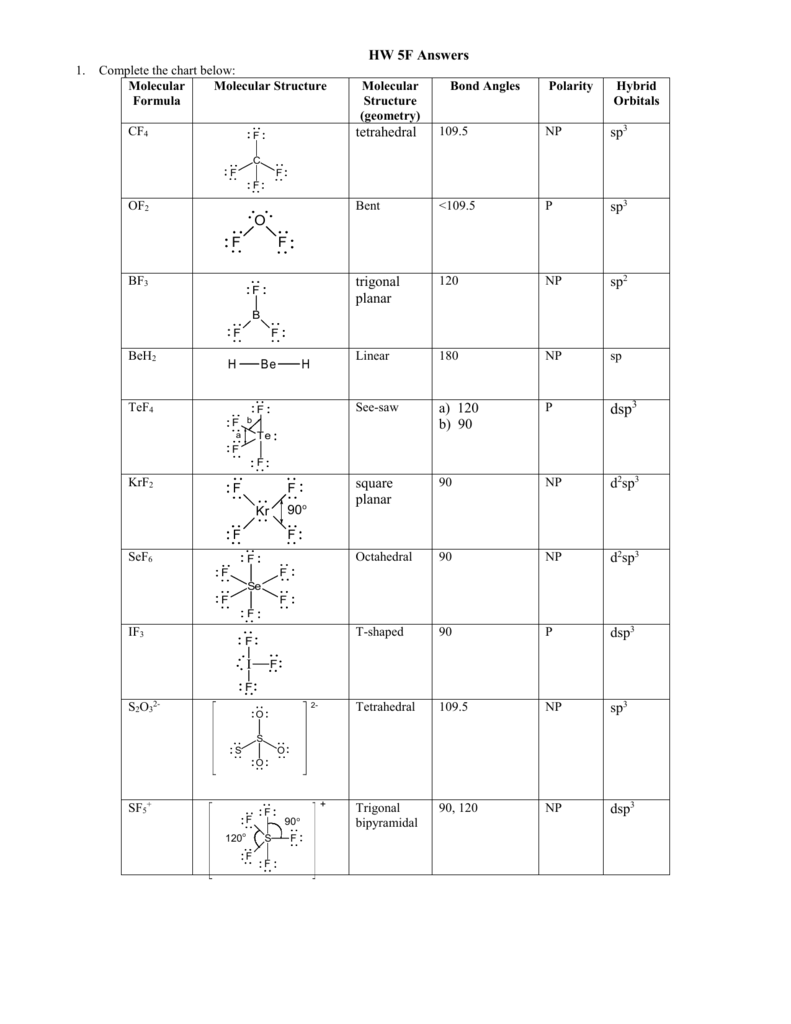
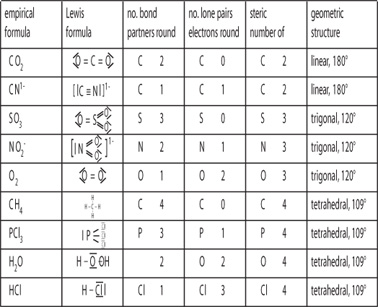
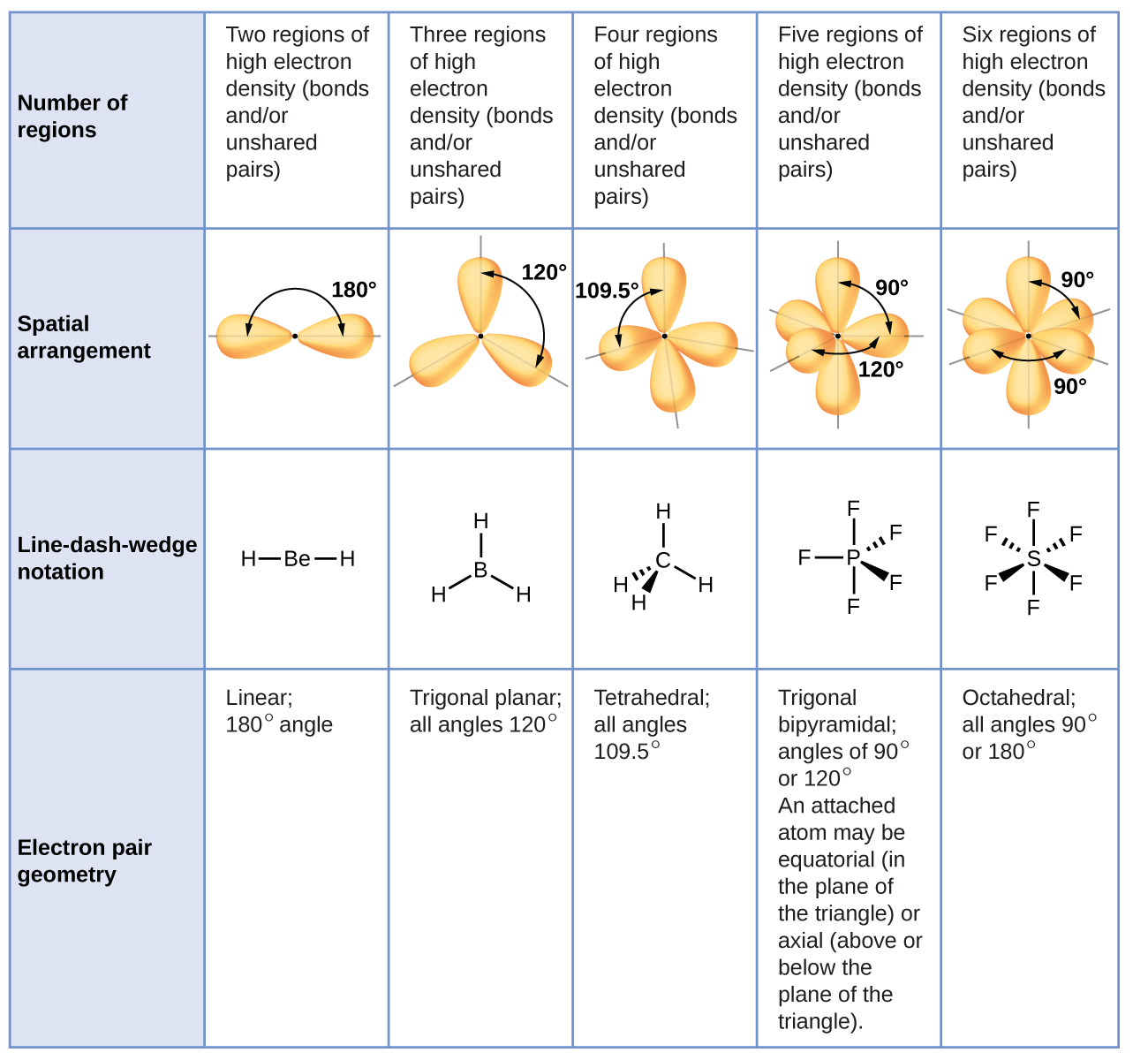
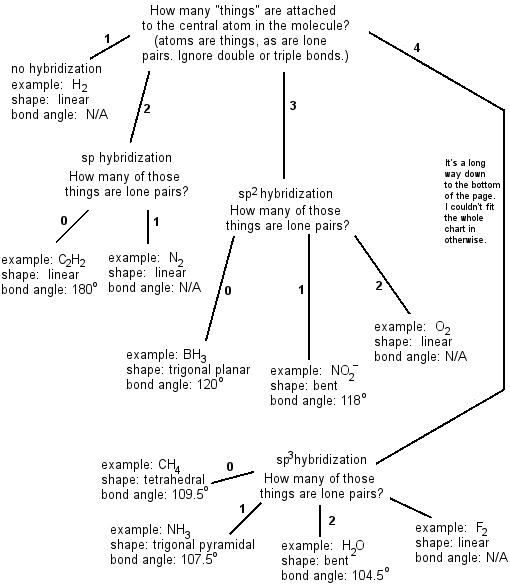
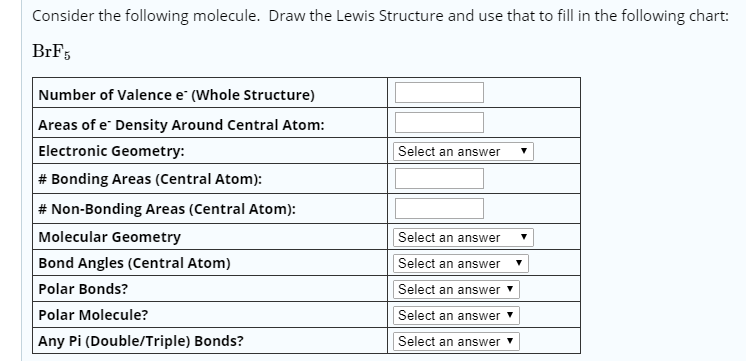
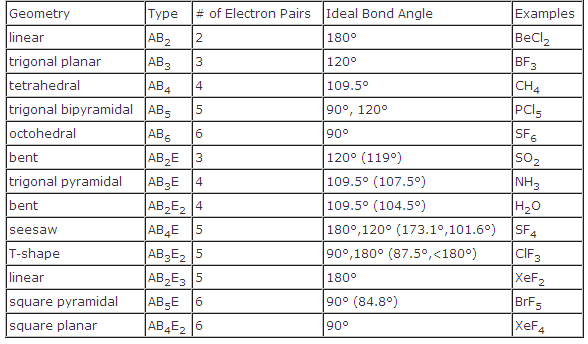
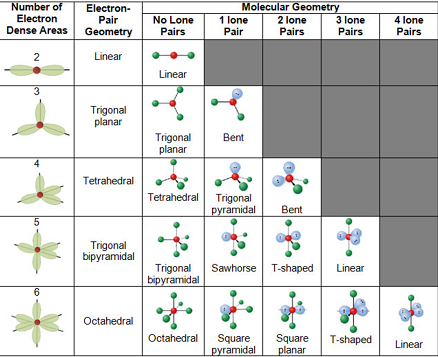
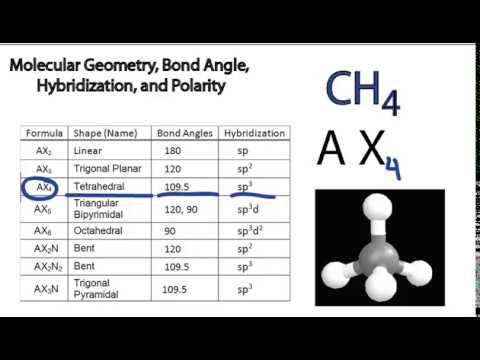
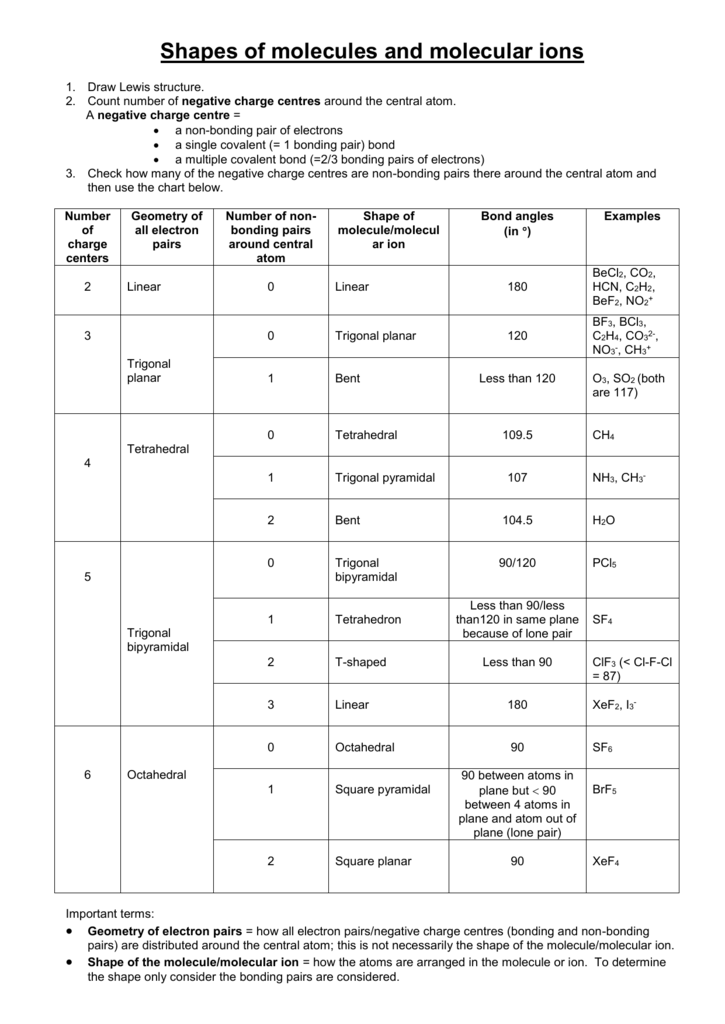
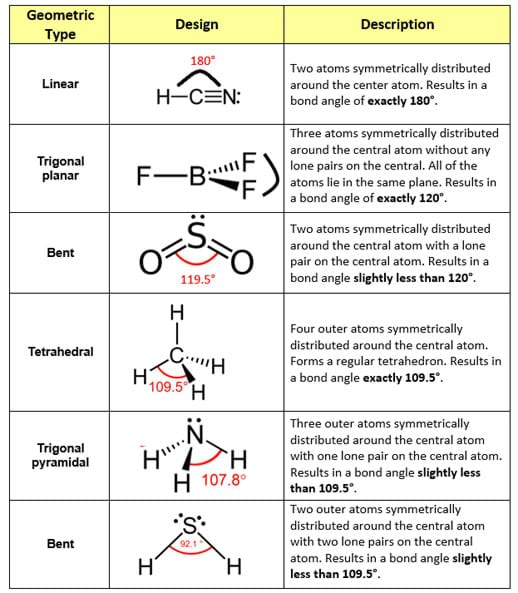
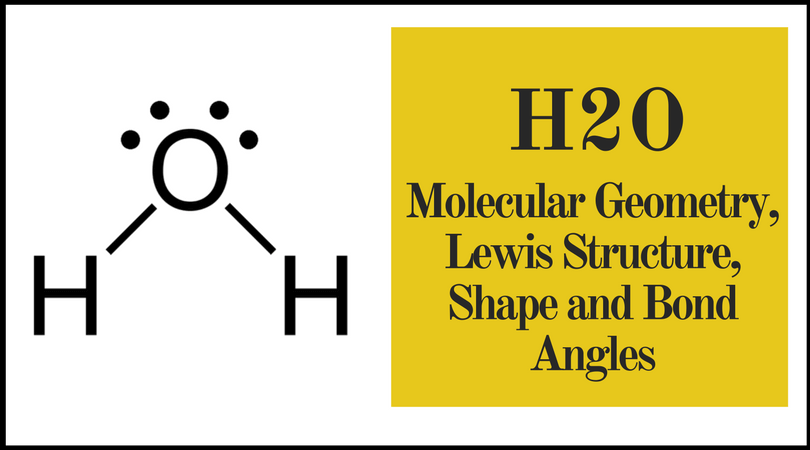
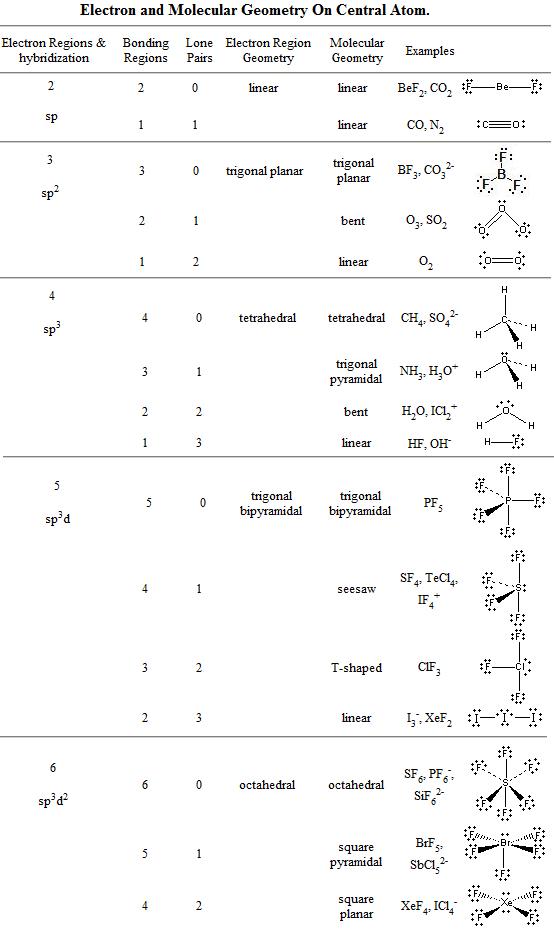
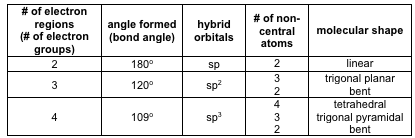
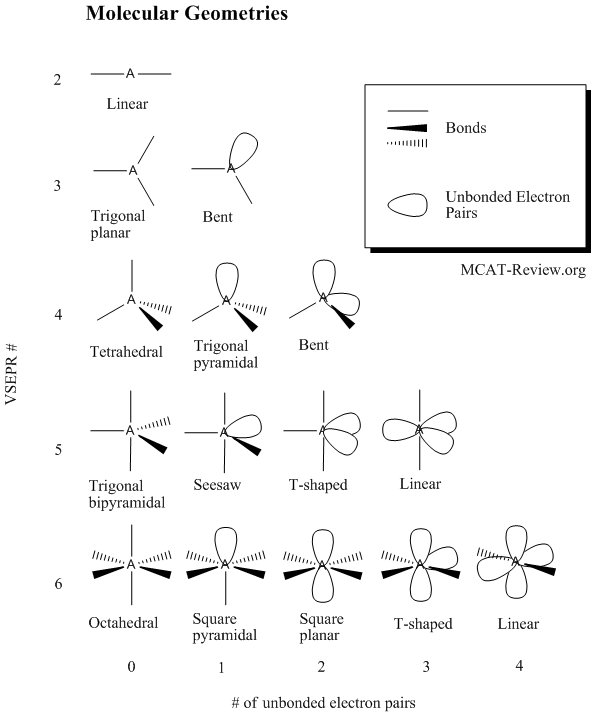
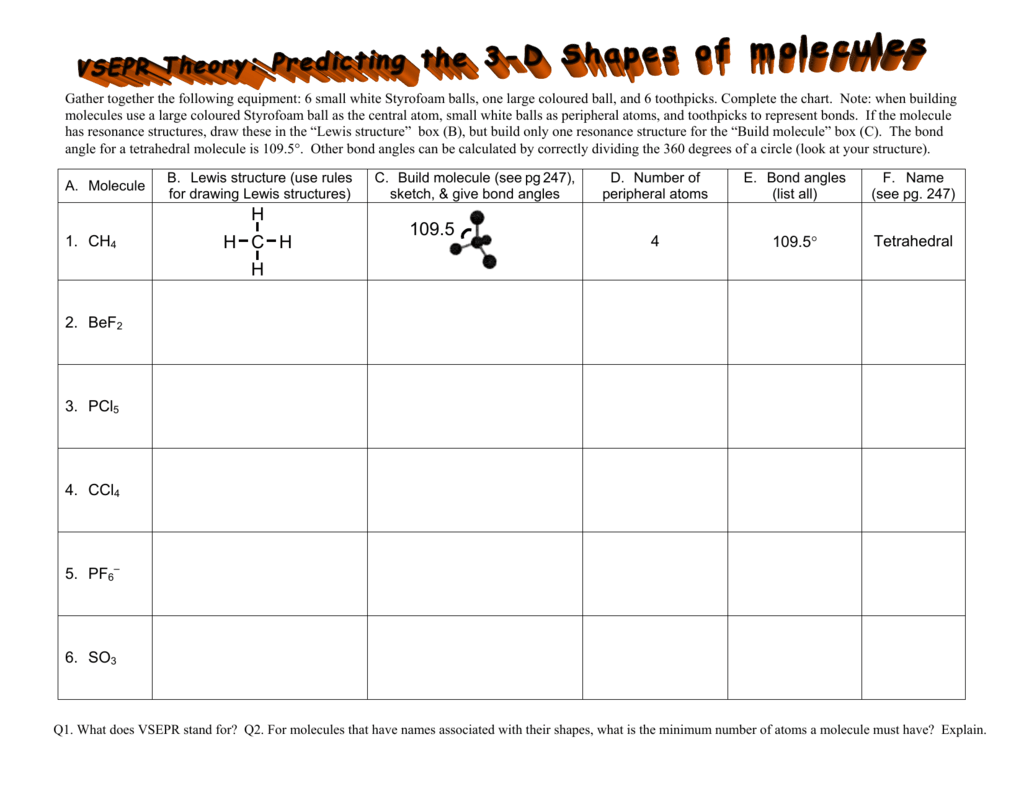
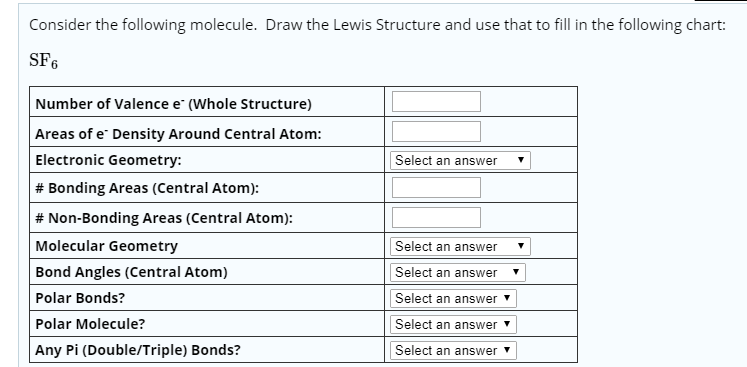
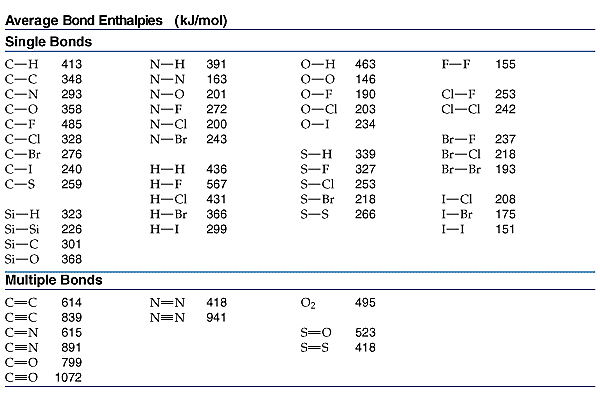
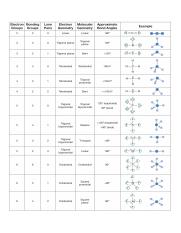
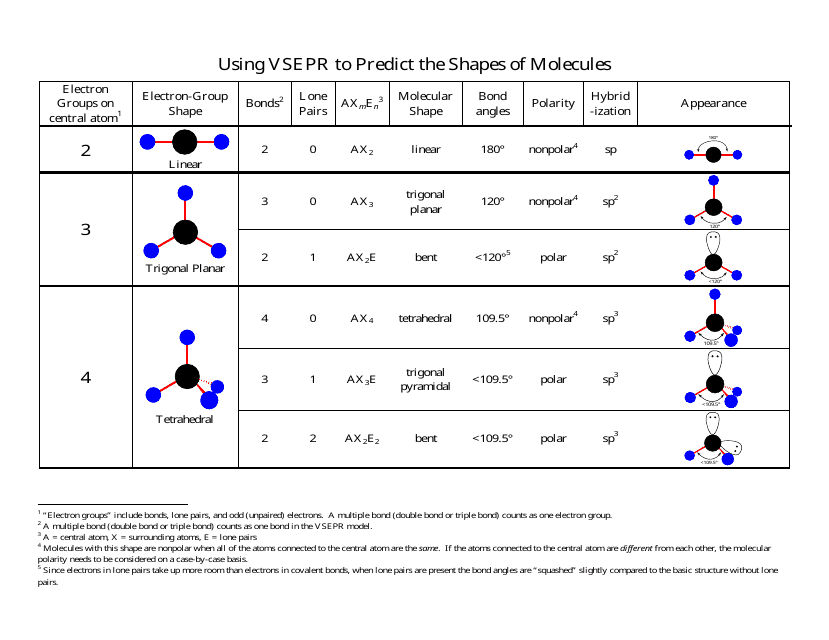
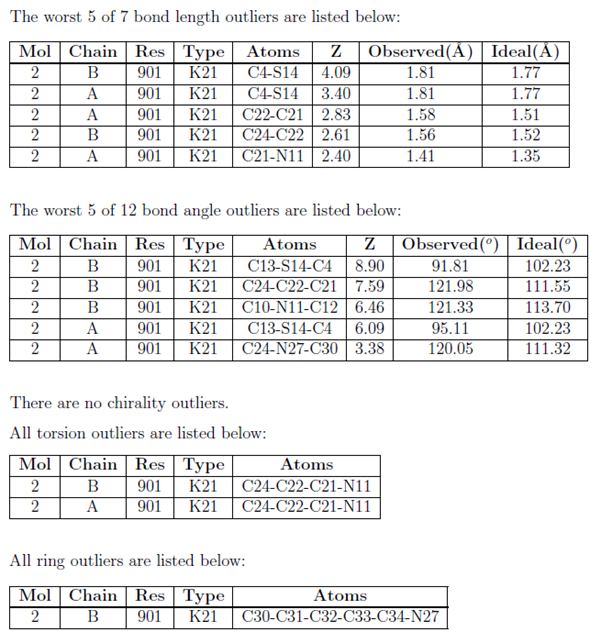
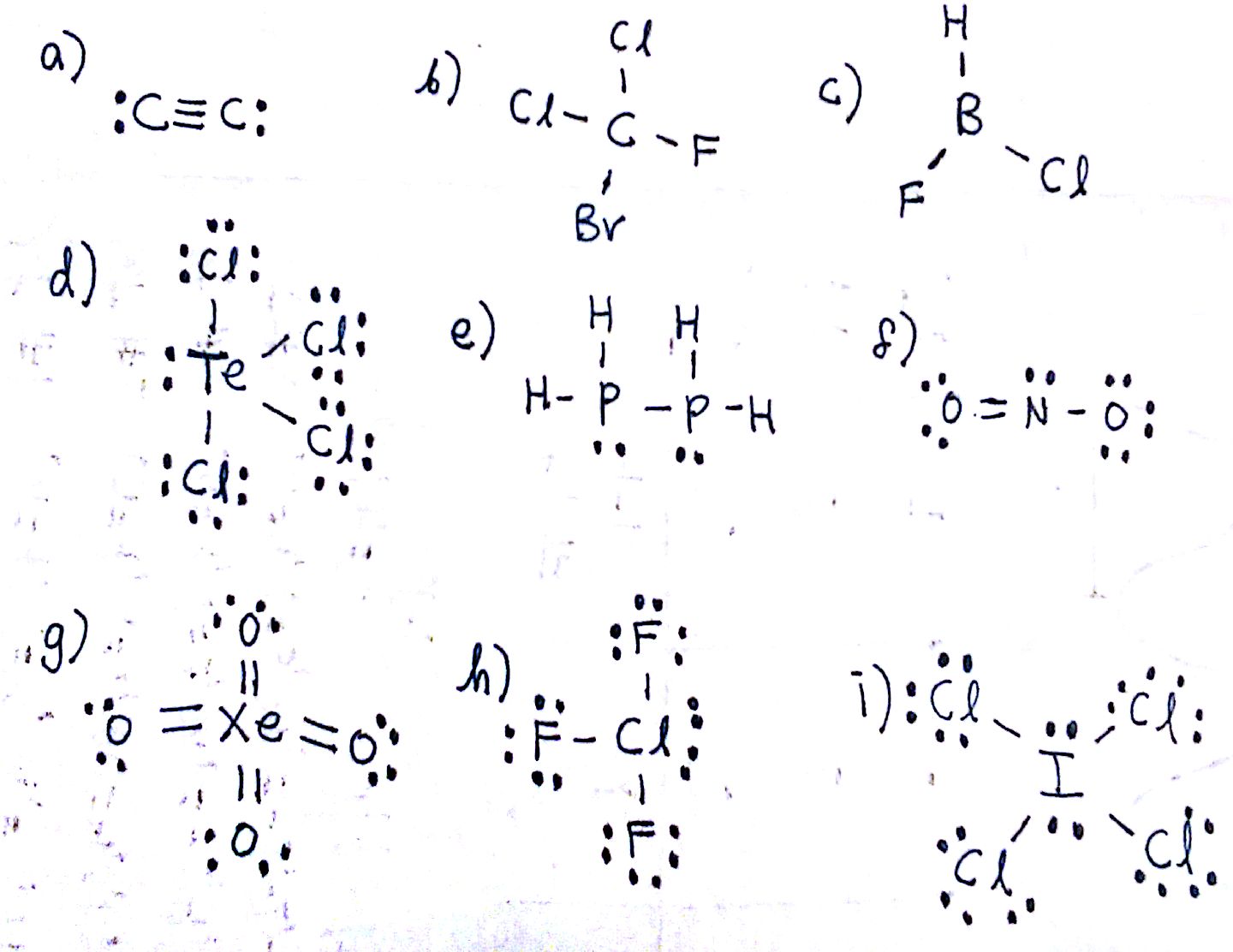Molecular Bond Angles Chart
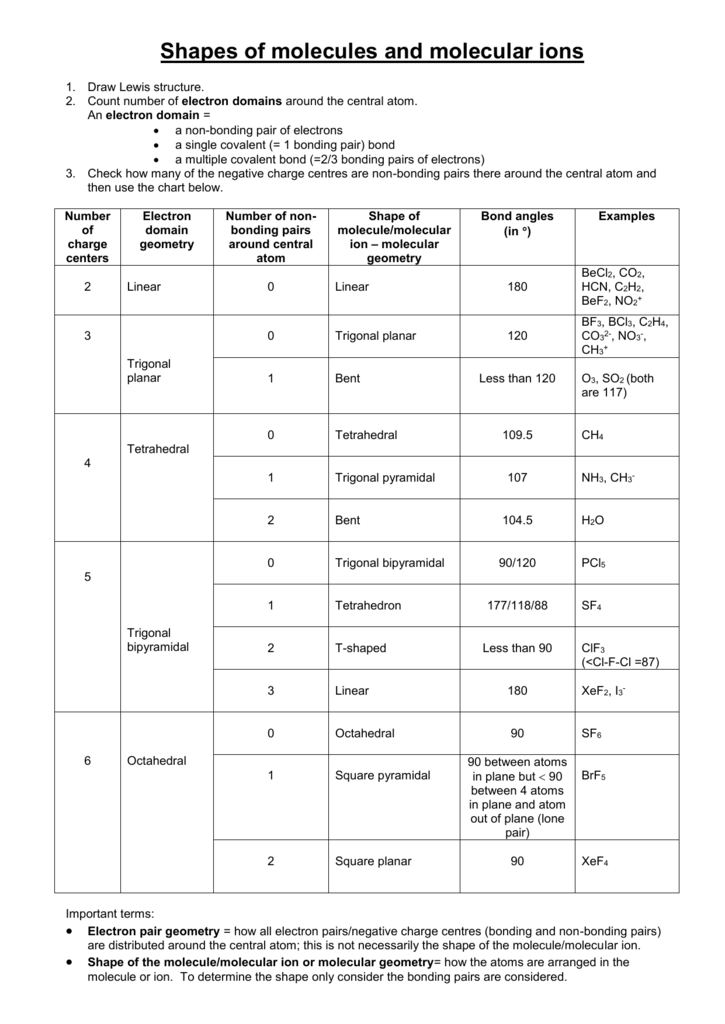
Its bond angles are 90 and 120 where the equatorial equatorial bonds are 120 apart from one another and all other angles are 90.
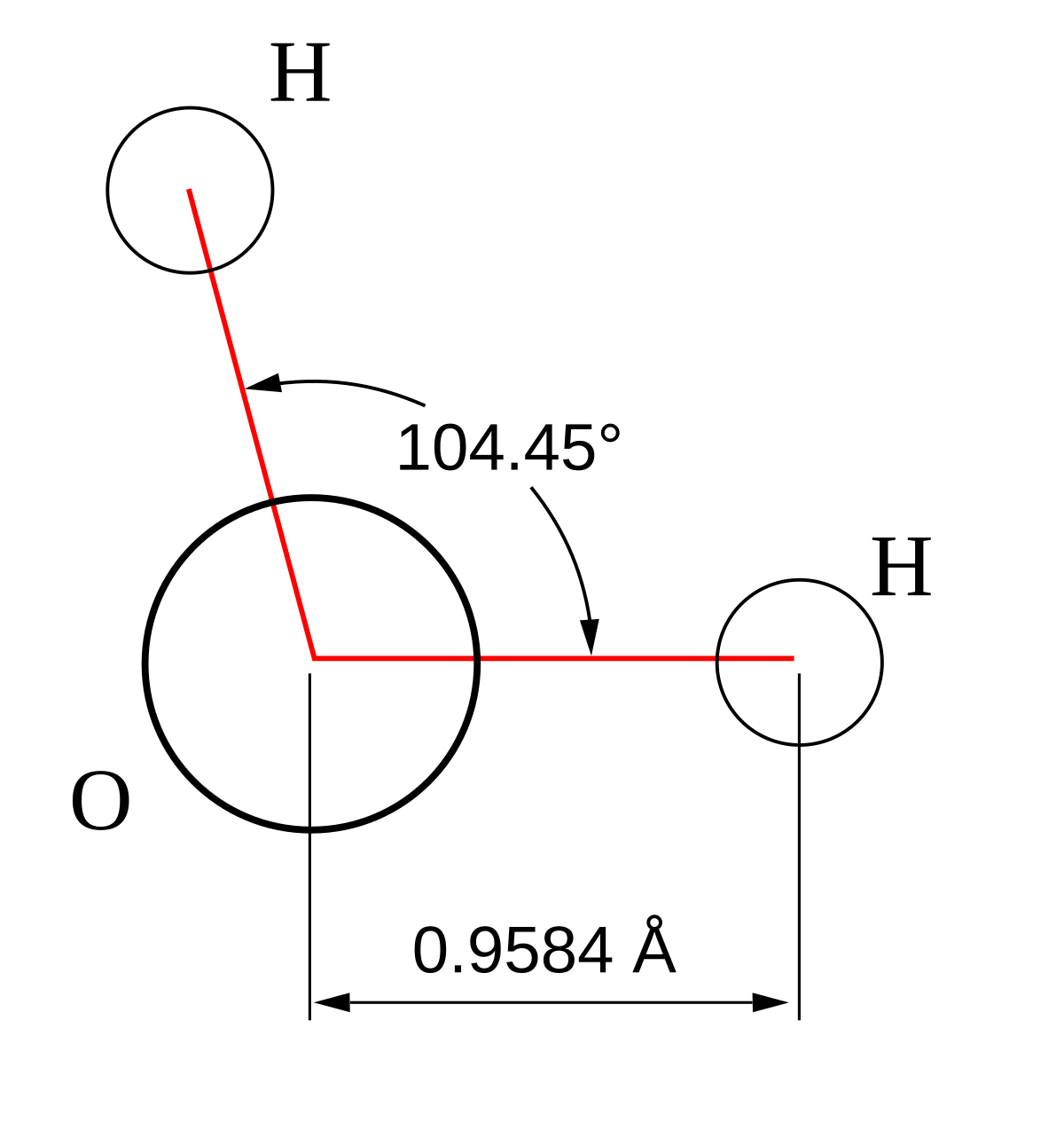
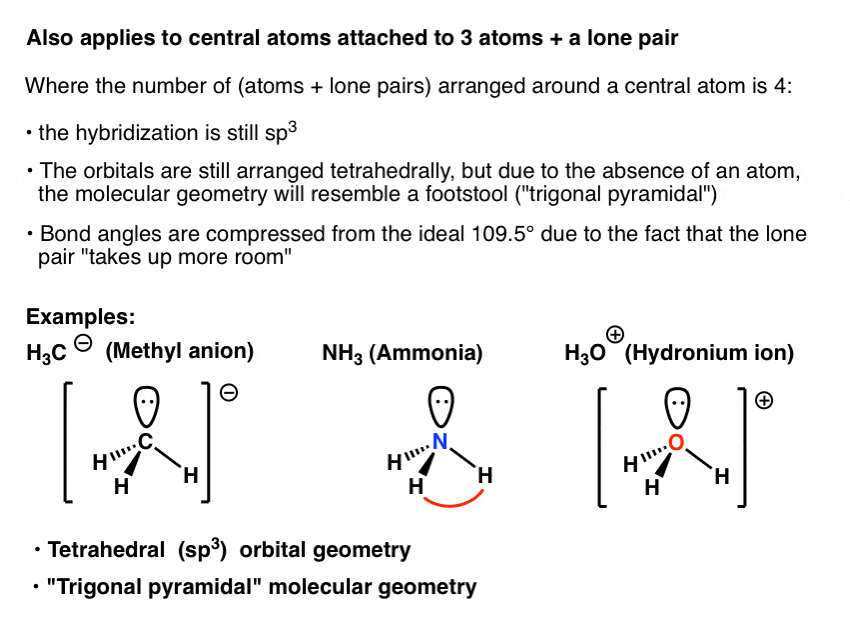
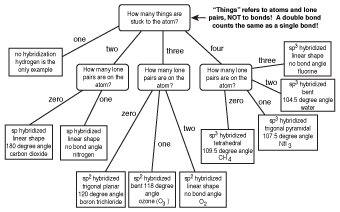
Molecular bond angles chart. Five atoms around the central atom. Four bonds on one central atom with bond angles of 109 5. For bent molecular geometry when the electron pair geometry is tetrahedral the bond angle is around 105 degrees.
Bond angles are now less than 109 5 ab 2 e 2. The most convenient way is shown here. We can draw the lewis structure on a sheet of paper.
4x4 5 19 electronic group geometry. 90 120 polar has a dipole moment. Total of electrons.
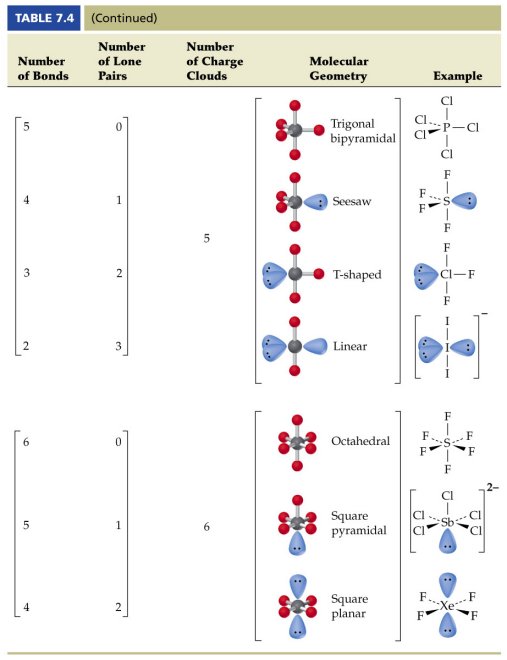
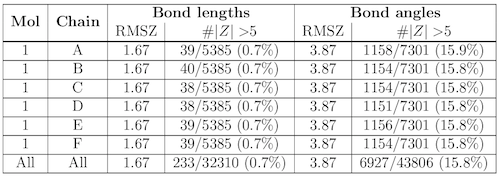
Ortep diagram is shown in figure 2 and selected bond lengths and angles are displayed in table 1. Seesaw start with ab 5. Three in a plane with bond angles of 120 and two on opposite ends of the molecule.
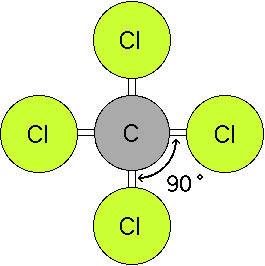
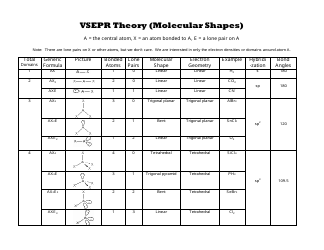
Triangular and in one plane with bond angles of 120. Lets consider the lewis structure for ccl 4. Molecular shape electron geometry example hybridi zation bond angles ax 5 5 0 trigonal bipyramid trigonal bipyramid asf 5 ax 4e 4 1 see saw trigonal bipyramid seh 4 ax 3e 2 3 2 t shape trigonal bipyramid icl 3 5 ax 2e 3 2 3 linear trigonal bipyramid brf 2 sp3d 90 and 120 ax 6 6 0 octahedral octahedral secl 6 ax 5e 5 1 square pyramid octahedral.
Mof6 the molybdenum fluoride mof6 oc 6 11. Bent start with ab 4 molecule tetrahedral and replace 2 b atoms with 2 lone pairs lone pair electrons repel each other and the bonding electrons bond angles are now less than 109 5 molecular geometries from trigonal bipyramidal electron domain geometry ab 4 e.